
Researchers at the University of Galway have made a significant advancement in bioprinting by creating tissues that mimic the shape-changing behaviors of biological tissues during organ development. This cutting-edge technology represents a major step toward generating functional bioprinted organs, with potential applications in disease modeling, drug screening, and regenerative medicine.
The research, led by the School of Engineering and the CÚRAM Research Centre for Medical Devices at University of Galway, has been published in Advanced Functional Materials. It focuses on replicating heart tissues using an innovative bioprinting technique that incorporates dynamic shape changes akin to those seen during natural embryonic development.
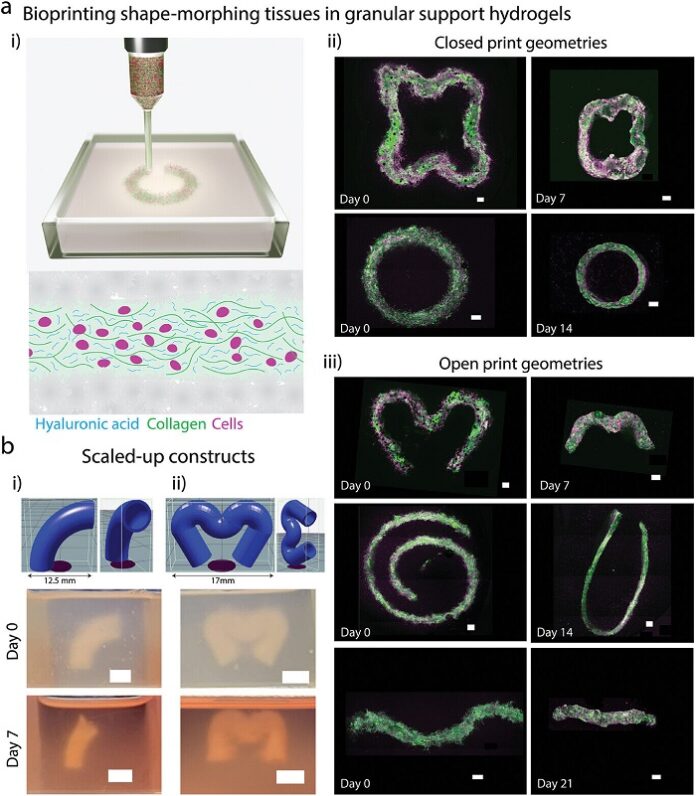
Bioprinting traditionally seeks to recreate the final structure of organs, such as the heart, but often neglects the critical role of shape-morphing processes that occur during development. For example, the heart begins as a simple tube that bends and twists to form its mature four-chambered structure. This morphing process is essential for the structural and functional development of heart tissues.
The Galway team developed a new platform using “embedded bioprinting” to produce tissues that undergo programmable, cell-driven 4D shape changes. The process improves the structural and functional maturity of bioprinted heart tissues, enabling stronger and faster contractions.
“Our work introduces a novel method to bioprint tissues that undergo predictable shape-morphing driven by cell-generated forces,” said lead author Ankita Pramanick, a CÚRAM Ph.D. candidate. “This approach enhances the structural and functional maturity of bioprinted heart tissues, advancing their potential for use in regenerative medicine.”
As reported by medicalxpress, the team demonstrated that cell-generated forces could guide tissue shape changes, which could be controlled by modifying the initial print geometry and bioink stiffness. These shape-morphing behaviors improved cell alignment and enhanced the contractile properties of the tissues. Additionally, a computational model was developed to predict the behavior of shape-morphing tissues.
Professor Andrew Daly, Associate Professor in Biomedical Engineering and principal investigator on the project, highlighted the importance of the findings: “Allowing bioprinted heart tissues to undergo shape-morphing enables them to beat stronger and faster. This is a significant step toward creating more mature bioprinted tissues in the lab that better replicate adult human heart structures.”
While this breakthrough represents a major advancement, challenges remain before functional bioprinted organs can be implanted in humans. Future work will focus on scaling the technique to human-sized hearts and integrating blood vessels to sustain larger constructs in laboratory settings.
Professor Daly added, “This research brings us closer to bioprinting functional organs with broad applications in cardiovascular medicine. Although there is much work to be done, our shape-morphing approach represents an exciting milestone in the journey toward generating lab-grown organs.”