Dr. Varsha Narayanan*
Abstract
At the start of 2021, two COVID vaccines have received approval for emergency use in India. The review of study data available on the safety and efficacy of these vaccines, and understanding their technological development along with rational of their approval, can help in achievement of a more effective vaccination drive. It is important at this stage that apprehensions and concerns on these vaccines are addressed scientifically in a balanced manner to improve vaccination coverage and success. A peek into what the near future holds also points towards India emerging as an effective global player in vaccines.
Keywords: COVID, vaccine, adenoviral vector, inactivated, immunogenicity, safety
Introduction
It is nothing short of a medical wonder that vaccines have been developed for Coronavirus Disease (COVID) in the same year as the global pandemic itself. India has received emergency use authorization for two COVID vaccines at the start of 2021: The adenovirus-vector vaccine from Oxford University and Astra Zeneca UK, being manufactured in India by Serum Institute, Pune as COVISHIELD, and the indigenous inactivated vaccine COVAXIN by Bharat Biotech, Hyderabad with Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV).[1] While this undoubtedly represents the relentless development and glory of medical science for India, there have also been several voices of apprehension and concern which need appropriate addressing as well as more data. However, reviewing the current clinical data available in public domain itself can considerably lay to rest some of the concerns, help in informed decision making, and play a reassuring role in the successful vaccination drive for India.
COVISHIELD
This is the vaccine being manufactured in India by Serum Institute, Pune and is the chimpanzee adenovirus-vector (ChAdOx1 nCoV-19) vaccine expressing the SARS-CoV-2 spike protein, developed by Oxford University and Astra Zeneca UK, called AZ1222. The data from phase 2 and 3 trials are peer reviewed and published in public domain. These studies used the meningococcal vaccine (MenACWY) as control.
The phase 2 study was conducted across 5 sites in UK with 1077 healthy adults aged 18-55 years (543 received ChAdOx1 nCoV-19 vaccine as a single dose of 5 × 10¹⁰ viral particles, 0.5ml intramuscularly, while the rest received the control vaccine).[2] There were protocol amendments of introducing prophylactic paracetamol in 2 of the sites, and 10 participants being assigned to a 2nd dose after 4 weeks. In the ChAdOx1 nCoV-19 group, spike-specific T-cell responses were seen to peak on day 14, and anti-spike IgG increased by day 28 (even further with the 2nd dose). Neutralizing antibody responses against SARS-CoV-2 evaluated by both plaque-reduction neutralization test (PRNT50) and microneutralization assay (MNA80), were detected in 100% and 91% respectively on day 28 post single dose, and 100% (MNA80) on day 42 in the 2-dose group.
Another phase 2 data set of 560 participants was thereafter released with 160 participants aged 18–55 years (100 in ChAdOx1 nCoV-19 group), 160 aged 56–69 years (120 in ChAdOx1 nCoV-19 group), and 240 aged ≥70 years (200 in ChAdOx1 nCoV-19 group).[3] The 2-dose regimen was studied in the 18-55 years ChAdOx1 nCoV-19 groups (as the single dose regimen had already been evaluated in the first phase 2 study in this age group) while in the >55 years age groups, both single dose and 2-dose regimens in a 1:1 ratio were studied. In all 2-dose regimen ChAdOx1 nCoV-19 groups, low (half) dose-standard dose as well as the standard dose-standard dose regimens were evaluated. This study confirmed that antibody responses against the SARS-CoV-2 spike protein were induced in all age groups (including ≥70 years) and were further increased and maintained at 28 days after the 2nd dose of vaccination. Cellular immune responses were also induced in all age and dose groups, peaking at day 14 after vaccination. Within each age group, no significant differences were seen in neutralization antibody titers between low-dose and standard-dose vaccine recipients which were achieved by 14 days post 2nd dose in >99% participants (MNA80).
Local and systemic reactions were seen in 60-80% participants in the ChAdOx1 nCoV-19 group (20-30% higher than the control arm), which were reduced by 10-15% by using prophylactic paracetamol.[2] Local side effects were mainly injection site pain-tenderness of mild to moderate intensity. Systemic reactions were mainly fatigue and headache, along with muscle aches, malaise and fever, with the intensity being highest in first 24 hours post injection and resolution thereafter. Local and systemic reactions were reported in 88% and 86%, 73% and 77%, and 61% and 65% in the 18–55 years, 56–69 years and ≥70 years age groups respectively, thereby displaying reduced reactogenicity in the elderly.[3] Among 13 serious adverse events occurring during the study period, none were vaccine related.
The phase 3 study has been conducted across 40,000 adult (≥18 years) participants in UK, Brazil and South Africa, with the data of 11,636 (ChAdOx1 nCoV-19 group + MenACWY control group) published in peer reviewed public domain.[4] Participants in the ChAdOx1 nCoV-19 group received two doses containing 5 × 10¹⁰ viral particles, 0.5ml intramuscularly (standard dose; SD/SD group; N=4440: 2377 UK and 2063 Brazil), while a subset received half (low) 1st dose and standard 2nd dose (LD/SD group; N=1367 UK). The 2nd dose was given from 4-12 weeks apart. Efficacy was assessed based on non-occurrence of symptomatic COVID-19 in seronegative participants (with nucleic acid amplification test-positive swab) after 14 days of the 2nd dose.
Efficacy was seen to be 62.1% (SD-SD group) and 90% (LD-SD group) with overall efficacy of 70.4%. Symptomatic COVID infections were seen to occur in 0.5% vs 1.7% in the vaccine vs control group (0.2% vs 2.2% in LD-SD group; 0.6% vs 1.6% in SD-SD group). While the control group had 10 and 2 cases of hospitalized and severe COVID respectively (with 1 death) >21 days post 1st dose, none such cases were seen in the ChAdOx1 nCoV-19 vaccine group. The regulatory approval for emergency use in UK has been given to the SD-SD dosing regimen which had satisfactory safety, efficacy (>50%), immunogenicity, sample size and age-group representation. The LD-SD group had inadequate sample size as well as absence of participants >55 years (10-12% in SD-SD group). The rate of serious adverse events was 0.7% in the ChAdOx1 nCoV-19 group (vs 0.8% in MenACWY control group). There were 3 cases of transverse myelitis reported in ChAdOx1 nCoV-19 group with only one case concluded as vaccine related, 14 days after the 2nd dose.
A bridging (phase 2/3) observer-blind randomized control immunogenicity-safety study has been performed in India across 14 centers in 1600 healthy adults ≥18 years.[5] 400 of these participants are in the group receiving COVISHIELD or ChAdOx1 nCoV-19 by Oxford-Astra Zeneca in a 3:1 ratio to assess immunogenicity. The remaining 1200 are in the group receiving COVISHIELD or placebo in a 3:1 ratio to assess safety. Dosage regimen is the recommended 0.5ml intramuscularly 28 days apart. The recruitment for the study was completed in November 2020, and the results are not yet in public domain as on mid-January 2021. This data is being awaited as it can shed light on how COVISHIELD fares in India in terms of safety-tolerance and immunogenicity, even though the same has been confirmed in global trials.
COVAXIN
Among the conventional technology vaccines, India’s indigenous vaccine COVAXIN (BBV152 in clinical trials), is a result of the collaborative effect of Bharat Biotech-ICMR-NIV. It is a whole-virion vaccine consisting of the killed-inactivated NIV-2020-770 SARS-CoV-2 strain isolated from a COVID-19 patient, sequenced at ICMR-NIV and manufactured at Bharat Biotech’s Bio-safety level 3 facilities. It is produced in Vero cell lines and is formulated with a Toll-like receptor 7/8 agonist molecule (IMDG) adsorbed to alum (Algel). The results of phase 1 and phase 2 clinical trials are in public domain but awaiting peer review.
The phase 1 was a double-blind randomized controlled study to evaluate the safety and immunogenicity in 375 volunteers (100 each in the 3ug and 6ug dose Algel-IMDG arms, 100 in the 6ug dose Algel only arm, and 75 in the Algel only control arm) as a 2-dose intramuscular injection (0.5ml/dose) regimen, given 2 weeks apart.[6] While majority of trial participants showed no reaction, some had mild events with all showing resolution. Immune response with both neutralizing antibodies (anti-IgG S1) and Th-1 cell mediated response was detected.
The phase 2 was carried out as a double-blind, randomized, multicenter trial with both 3ug and 6ug Algel-IMDG formulations, given as 2 doses 4 weeks apart, in 380 patients (12-65 years: 190 per dosage arm).[7] The corresponding phase 1 participants in the 3ug and 6ug trial arms (100 each) were also followed up. The primary outcome of seroconversion (≥4-fold above baseline rates of neutralizing antibodies, 4 weeks after the 2nd dose were as follows: 3 ug – 92·9% (PRNT50) and 88% (MNA50); 6ug – 98·3% (PRNT50) and 96.6% (MNA50). No significant differences were observed in seroconversion rates (SCR) or geometrical mean titers (GMTs) across the three age groups of 12-18, 18-55 and 55-65 years, and for both spike S1 protein- receptor-binding domain RBD, and nucleocapsid N protein of SARS-CoV-2. The GMTs were 2-fold greater than seen in corresponding phase 1 trial arms, with the participants showing seroconversion rates of 73·5%, and 81.1% (MNA50) for the 3µg and 6ug groups respectively 3 months post 2nd dose. There was also a significant increase in Th1-biased cytokines, such as IFN-γ, IL-2 and TNF-α on day 56, suggesting a robust cell mediated immune response as well.
Apart from this vaccine showing high rates of immunogenicity in phase1/2, there is also satisfactory evidence of safety and tolerance, with an overall reaction rate of around 10% after each dose (4% local and 6% systemic reactions) and no serious adverse reactions after 2 vaccine doses. Local adverse effects were pain, itching, redness, or stiffness/weakness in injected arm, while systemic ones included fever, headache, body ache, and malaise/weakness. These are similar to reactions seen with all vaccines, and were mild to moderate, with most resolving in 24 hours. There was no difference in the rates between the first/second dose, or between the 3/6ug dose.
The 6 µg Algel-IMDG-induced responses were comparable to those observed in convalescent serum collected from patients who had recovered from COVID-19, and this formulation has been taken ahead for phase 3 trials, and also given conditional approval for emergency use. The phase 3 multicentric trial is underway with recruitment completed at 25,800, with most of them having received the 1st dose, and >5000 of them having also received the 2nd dose without reports of any significant adverse events.[8] The final efficacy data related to COVID prevention post 2 doses, is expected in March-April 2021.
The regulatory emergency approval for COVAXIN (given in the form of a clinical trial mode) before phase 3 completion has raised concerns. The aspects to be considered here are firstly that India being one of the largest population segments of the world would require an unprecedented manufacturing of vaccine dosages which can be best achieved with development of more than one vaccine. Secondly COVAXIN has shown both high immunogenicity in phase 2, which is considered a surrogate marker of vaccine efficacy, as well as high safety in both phase 2 and initial phase 3 studies of almost 25,000 patients.[1,7,8] The generation of data that actually confirms not getting a disease as the efficacy end point requires long term study in very large populations, which could be a crucial factor in the control of an ongoing pandemic. The New Drugs and Clinical Trials Rules 2019 has the provision for investigational drugs for unmet medical needs of serious and life-threatening diseases in the country to be approved based on remarkable phase 2 data with a definite dose, along with a continuation of studies to verify clinical benefits in larger populations.[9] Thirdly, due to the presence of multiple antigens including the less variable N protein present in a whole virus inactivated vaccine, the theoretical possibility of a back-up for variant strains has been considered.[10,11] Therefore, in the light of these points, the emergency conditional authorization of this vaccine may be of potential benefit in the success of the vaccination drive in India.
Table 1: Phase 2 results for COVAXIN and AD1222 (manufactured as COVISHIELD, India)
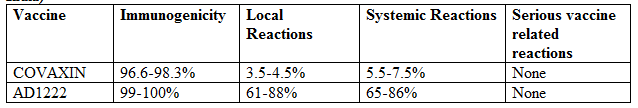
COVAXIN (BBV152: 6ug Algel-IMDG) and AD1222 (ChAdOx1 nCoV-19 as 5 × 10¹⁰ viral particles) was given as 0.5ml muscularly, two doses 4 weeks apart.
Immunogenicity for COVAXIN was assessed by Seroconversion rate (SCR) defined as ≥4-fold above baseline rates of neutralizing antibodies evaluated by plaque-reduction neutralization test (PRNT50) and microneutralization assay (MNA50) at 28 days post 2nd dose.
Immunogenicity for ChAdOx1 nCoV-19 was assessed by PRNT50 assay, which determined the extent to which serum can be diluted and still reduce SARS-CoV-2 plaque formation by 50% at day 28, and MNA80 assay, with titers inducing 80% virus neutralization.
Local reactions are those at injection site mainly pain-tenderness, and systemic reactions include fever, headache, body ache, and malaise. For Covaxin local (4.2%-1st dose; 3.7%-2nd dose) and systemic (7.4%-1st dose; 5.8%-2nd dose) reaction rate was similar after the 1st and 2nd dose.
Table 2: Phase 3 (interim) results of AZ1222 (ChAdOx1 nCoV-19)


N=5807 (ChAdOx1 nCoV-19) and 5829 (MenACWY control); 1 COVID related death was seen in control group
Authorized Vaccines in Other Countries
The efficacy rates of mRNA COVID vaccines, from Pfizer/BioNTech and Moderna, reported in peer reviewed publications, has been high at 95% and 94.1% respectively.[12,13] Given their more stringent storage temperature requirements (compared to the regular refrigerator 2-8 deg C storage of the adenoviral-vector and inactivated vaccines), and absence of initiating bridging studies in India may preclude their availability anytime soon, however several Indians are receiving the same in the countries of approval like USA, UK and Canada. Also, discussion for collaborations and studies for bringing the mRNA vaccines from Pfizer and Moderna to India are still ongoing and a future possibility.
SPUTNIK V Adenoviral (Ad26/5) vector vaccine authorized in Russia and developed by the Gamaleya National Research Center for Epidemiology and Microbiology of the Ministry of Health of the Russian Federation has shown an efficacy of 91.4%, based on the second interim analysis of data obtained 28 days after administering the 1st dose (7 days after the 2nd dose). The preliminary data from volunteers obtained 42 days post 1st dose (21 days post 2nd dose) is indicating >95% efficacy without any unexpected adverse events.[14] While this data is awaiting peer review, this vaccine is being developed and undergoing clinical trials in India in collaboration with Dr Reddy’s Labs (DRL), and is being expected later in 2021.
The results of the completed Phase 3 study across 60,000 volunteers of Sinopharm’s inactivated vaccine (approved in China and UAE where already in regular use), published in a press release (not yet peer reviewed) has claimed an efficacy of around 79% in China and 86% in the UAE.[15] Again, the inactivated vaccines from China would not be expected in India given the availability of its own indigenous inactivated vaccine.
Future Prospects
So far from existing data of recovering patients as well as vaccine trial extrapolations, duration of conferred immunity with COVID vaccines stands at around at least 6 months with the cell mediated immunity expected to last longer than the humoral component.[16] India is initiating its vaccination drive starting with health care and front-line workers, elderly and those with comorbidities or risks, and thereafter the general adult population.
There are several vaccines in the pipeline globally and in India.[17] Apart from Sputnik V (with DRL), Cadila Healthcare has developed a Novel Corona Virus-2019-nCov-Vaccine using DNA platform technology and has got approval to conduct phase 3 study in India.[1]
Conclusion
In all India can look forward to initiating successful vaccination and control of COVID in 2021 with 2 domestically manufactured vaccines, and also with more vaccines in the pipeline. The decade ahead for India promises to be one of medical advancements and disease control paralleling several developed countries, as well as emergence as a global player in development and supply of vaccines.
References:
- Ministry of Health and Family Welfare [Internet]: Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine. Posted: 03 JAN 2021 11:23AM by PIB Delhi. [cited 2021 Jan 5]. Available from: https://pib.gov.in/PressReleseDetail.aspx?PRID=1685761
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet 2020 Aug 15;396:467–78.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020;396:1979–93.
- Voysey M, Clemen SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet [internet]. Published online 2020 December 8; S0140-6736(20)32661-1. [cited 2021 Jan 5]. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
- A Phase 2/3 Observer-Blind Randomized Controlled Study to determine the Safety and Immunogenicity of COVISHIELD (COVID-19 Vaccine) in healthy Indian adults. CTRI/2020/08/02170. [cited 2021 Jan 5]. Available from: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V et al. A Phase 1: Safety and Immunogenicity Trial of an Inactivated SARS-CoV-2 Vaccine-BBV152. 2020 December 15. Pre-Print. [cited 2021 Jan 6]. Available from: https://www.medrxiv.org/content/10.1101/2020.12.11.20210419v1
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S et al. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomized controlled trial) and the persistence of immune responses from a phase 1 follow-up report.2020 December 22. Pre-Print. [cited 2021 Jan 6]. Available from: https://www.medrxiv.org/content/10.1101/2020.12.21.20248643v1.full.pdf
- Das S. Covid-19 vaccine: Bharat Biotech completes recruitment for phase 3 trials [internet]. Business Standard. 2021 January 7. [cited 2021 Jan 8]. Available from: https://www.business-standard.com/article/current-affairs/covid-19-vaccine-bharat-biotech-completes-recruitment-for-phase-3-trials-121010701171_1.html
- CDSCO [internet]. New Drugs and Clinical Trials Rules 2019. Part II, Sec 3(i) Accelerated Approval Process Page 186. [cited 2021 Jan 9]. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDrugs_CTRules_2019.pdf
- Ahlén G, Frelin L, Nikouyan N, Weber F, Höglund U, Larsson O et al for the OPENCORONA Consortium. 2020. The SARS-CoV-2 N protein is a good component in a vaccine. J Virol [Internet] 94:e01279-20. [cited 2021, Jan 9]. Available from: https://jvi.asm.org/content/94/18/e01279-20
- Ghosh P. Bharat Biotech vaccine may have some advantage [internet]. Hindustan Times. 2021 January 03. [cited 2021 Jan 9]. Available from: https://www.hindustantimes.com/india-news/bharat-biotech-vaccine-may-have-some-advantages-icmr-chief-on-covaxin-mutant-strain/story-wPtilHX022GmdfxF4tSxaM.html
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockart S et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med Dec 2020; 383:2603-15.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. NEJM [internet]. Published online 2021 Dec 30. [cited 2021 Jan 5]. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2035389
- Sputnik vaccine [internet]. Sputnik V– Second Interim Analysis of clinical trial data. Press Release. 2020 Nov 24. [cited 2021 Jan 9]. Available from: https://sputnikvaccine.com/newsroom/pressreleases/second-interim-analysis-of-clinical-trial-data-showed-a-91-4-efficacy-for-the-sputnik-v-vaccine-on-d/
- Reuters [internet]. China gives its first COVID-19 vaccine approval to Sinopharm. Press Release. 2020 Dec 31. [cited 2021 Jan 5]. Available from: https://www.reuters.com/article/us-health-coronavirus-vaccine-china/china-gives-its-first-covid-19-vaccine-approval-to-sinopharm-idUSKBN29505P
- Swell HF, Agius RM, Kendrick D. Covid-19 vaccines: delivering protective immunity. BMJ [internet]. 2020;371:m4838. [cited 2021 Jan 9]. Available on: https://www.bmj.com/content/371/bmj.m4838
- Rawat K. Kumari P, Saha L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021 Feb 5;892:73751.
*Consultant Family Medicine and Holistic Health, Dr Varsha’s Health Solutions, Mumbai. Email: info@drvarsha.com