ABSTRACT
Objective: Antihypertensive drug combinations are being increasingly used in the man management of hypertension. This was an observational study to gather data about the real-world experience on the safety and efficacy of bisoprolol and amlodipine combination in the treatment of stage 2 essential hypertension.
Methods: 801 patients with stage 2 essential hypertension who fulfilled inclusion and exclusion criteria were enrolled in the study. They received fixed dose combination of AmJodipine (5 mg) and Bisoprolol (5 mg) OD for a period of 4 weeks. Patients were termed as “responder”. if the diastolic BP and systolic BP was < 90 mmHg and < 140 mmHg. respectively at the end of the trial period. The global efficacy and global tolerability was judged at the end of
4-week treatment period. Adverse events, either spontaneously reported by the patient. or noticed by the physician during trial period, were recorded. Statistical analysis was done us ing Paired test and Me Nemar’s test, as applicable.
Results: Out of the 801 patients enrolled in the study, 749 patients completed the study (median age 53.6 yrs). The mean systolic blood pressure at baseline was 171.9 ± 17.9 mm Hg. This reduced significantly (p < 0.001) to 152.9 ± 16.4 mmHg, 142.1 ± 13.1 mmHg and 134.3 ±
10.1 mmHg at the end of 1,2 and 4 weeks of treatment respectively. The mean diastolic blood pressure at baseline was 103.9 ± 9.6 mmHg. This reduced significantly (p < 0.001) to 93.5± 8.8 mmHg, 88 ± 7.3 mmHg and 83.4 ± 6.2 mmHg at the end of 1.2 and 4 weeks of treatment respectively. The mean heart rate at baseline was 83.3 ± 9.6 beats per minute. It reduced significantly (p < 0.0001) to 78.3 ± 7.2. 75.8 + 6.8 and 74.6 ± 6.8 beats per minute, at the end of the 1. 2 and 4 weeks of treatment respectively. The responder rate at the end of 4 weeks of treatment was 82.5. Excellent to good efficacy and tolerability was observed in 91.4% and 90.3% of the subjects, respectively.
Conclusion: Once daily administration of the ftxed dose combination of amlodipine 5 mg and bisoprolol 5 mg is effective, safe and well tolerated in treatment of patients suffering from stage 2 essential hypertension. (The Ind. Pract. 2008; 61(4):225–234)
KEYWORDS
fixed dose combination, Bisoprolol, Amlodipine, Essential Hypertension.
INTRODUCTION
The recent ESC/ESH guidelines for the management of hypertension state that monotherapy allows to achieve BP target in only a limited number of hypertensive pa tients’ and that ‘use of more than one agent Is necessary to achieve target BP in the ma jority of patients. 1 The JNC VU recommends that when the blood pressure is more than 0/10 mmHg above goal, consideration should be given to initiating therapy with 2 drugs, either as separate prescriptions or in fixed dose combination. 2
It has been well demonstrated in multiple studies that the response rate to any single class of antihypertensive agent, given as monotherapy, is approximately 45-55o/o. Thus, in approximately half of the hyperten sive population, a second drug will be required the randomized trials of antihyperten sive therapy conducted during the 1970s and 1980s, in which very large doses of single drug were employed as Initial therapy, more than 50o/o of patients required treatment with combination therapy in order to achieve the blood pressure goal indicated in the trial protocol. This implies that an in crease in the dose of the initial antihyper tensive agent does not usually significantly increase the effectiveness of monotherapy. It is well known that high doses of almost all antihypertensive agents increase the risk and th<.; severity of adverse effects. Adverse effects of drugs are the main cause of the low compliance of patients with antihyper tensive therapy in practice. Essential hyper tension has multiple mechanisms, and ra tional combination therapy allows interference with more than one of these mecha nisms. Rational combination therapy not only reduces adverse effects because of the use of low doses of each combined agent, but can also take advantage of counteractions that each of the combined agents can exert on the undesirable actions of the other agent.4
It has been shown that simplification of a drug regimen by using combination therapy in a single pill for hypertension resulted in significant increases in persistence with prescribed therapy.5
Both bisoprolol and amlodipine are widely used antihypertensive drugs. Bisoprolol being a beta-blocker (BB) Is well suited foe young patients who have a ‘vasoconstrictive’ type of hypertension with high renin while amlodiplne, a calcium channel blocker (CCB) is suited for older patients with ‘vol ume expanded’ type of hypertension and a low or suppressed renin activity. Hence, the physiologically and pharmacologically ra tional for the combination of these drugs is that they have complementary mechanisms of action and may counteract each others side-effects: for instance, CCBs tend to in crease heart rate (reflex tachycardia) BBs tend to lower the heart rate.
There is a clear paucity of scientific literature regarding the use of a combination of bisoprolol and amlodipine in the treatment of hypertension. The present observational study aims to gather data about the real world experience on the safety and efficacy of bisoprolol and amlodipine combination in the treatment of stage 2 essential hypertension.
OBJECTIVE
- To evaluate the efficacy of the fixed dose combination of bisoprolol (5 mg) and amlodipine (5 mg) for the treatment of stage 2 essential hypertension.
- To evaluate the responder rate to the fiXed dose combination of bisoprolol (5 mg) and amlodipine (5 mg) used for the treatment of stage 2 essential hypertension
- To evaluate the safety and tolerability of the fixed dose combination of bisoprolol (5 mg) and amlodipine (5 mg) for the treatment of moderate to stage 2 hypertension.
STUDY DESIGN
The study was an open, non-comparative, multicentric, and prospective trial conducted at 169 centres after obtaining a clearance from an independent ethics committee.
NUMBER OF PATIENTS
This study was conducted in 801 adult males and females newly diagnosed with a clinically confirmed stage 2 essential hyper tension (based on average of 2 or more re cordings each taken at 2 or more visits) and aged between 18 and 70 years. Patients with stage 2 hypertension uncontrolled by monotherapy with amlodipine or ramipril or atenolol were also recruited.
The patients were recruited after fulfill ing the inclusion and exclusion criteria mentioned below and after obtaining in formed written consent.
INCLUSION CRITERIA
- Patients of either sex aged 18 -70 yrs
- Newly diagnosed patients with clinically confirmed stage 2 hypertension (based on average of 2 or more recordings each taken at 2 or more visits) as per the JNC VII (Annexure 1)
- Patients with stage 2 hypertension un- controlled by monotherapy with amlodipine or ramipril or atenolol
EXCLUSION CRITERIA
- History of hypersensitivity to either of the components
- General contraindications of 13 – blockers and I or calcium channel blockers
- Concomitant illness such as uncontrolled diabetes mellitus. liver and I or kidney dysfunction, decompensated car diac failure.
- Alcohol and I or drug abuse.
- Pregnancy and I or lactation.
- Necessity for unavoidable concomitant drug therapy, which may vitiate interpretation of the results.
DRUG AND DOSAGE
Fixed dose combination of Amlodipine (5 mg) and Bisoprolol (5 mg).
1 tablet once daily (0.0.) preferably given in the morning. In case of inadequate BP control, at the end of the 2nd week of treatment. enalapril 5 mg can be added.
DURATION OF TREATMENT
The study was conducted for a period of 4 weeks.
DATA COLLECTION
Patients agreeing to participate in the survey were required to visit the hospital as per the 4 scheduled visits. The Physician had to capture the patient’s clinical history and perform a general examination (Pulse and BP measu rement) and administer the study medication during initial visit (VO). The patient has to continue the study medi cation containing amlodipine 5 mg plus Bisoprolol 5 mg. 1 tablet once daily in the morning during the entire study period (4 Weeks) and also Pulse and BP measurement will be recorded during the 3 scheduled fol low up visits V1 (Week 1). V2 (Week 2) and V3 (Week 4) ). In case of inadequate BP control, at the end of 2nd Week of treatment, enalapril 5 mg can be added. The Global im pression after usage of the study drug wilJ be recorded in the final Visit (V3). Physicians had to complete a clinical case report form for each patient that included information on the patient’s blood pressure, and con comitant medication usage.
Occurrence of any side effect suffered I reported by patient were also be noted at every visit.
ENDPOINTS
Efficacy
Patient were termed as “responder”, if the diastolic BP (DBP) and systolic BP of< 90 mm Hg and < 140 mmHg respectively was re corded at the end of trial period. The global efficacy was judged on a four point scale i.e. Excellent. good. satisfactory and poor. by the investigator at the end of 4 week treatment period based on the overall reduction in the systolic and diastolic blood pressure and the overall improvement in the patient’s condi tion.
Tolerability
The adverse events. either spontaneously reported by the patient, or noticed by the physician during trial period, were recorded. The global tolerability was judged on a four point scale i.e. excellent. good, satisfactory and poor, by the investigator at the end of 4- week treatment period.
Compliance
All medications were dispensed by the in vestigator to all eligible patients, during all visits. At each visit, patients were in structed to bring back the empty packs and all the unused medication, with a view to check compliance.
STATISTICAL ANALYSIS
Data on a continuous scale was expressed as Mean ± SD. while the frequency type of data was expressed as percentage. The dif ference from baseline to following visit is compared using Paired test. Repeated measure analysis of variance was used to see overall effect during trial period. All statistical test applied here were two tailed and p < 0.05 was considered as statistically significant.
Descriptive statistics are used to report the Demographics, Concomitant medica tion, Vital signs, Responder rate. Adverse Events and Global Expressions. Mean. Standard deviation. Minimum and Maximum is used for continuous variables and Frequency and Percentage is used for categorical variables. P-value is calculated by Comparing the average change of BP from Baseline to end of the study using paired t test with 2- tailed and 5o/o level of significance. P-value is calculated by Comparing the proportion of responder rate from Base line to e.1d of the study using Me Nemar’s test with 2- tailed and 5o/o level of significance.
RESULTS
A total of 801 patients were enrolled in the study and 749 patients completed the study. There were a total of 52 dropouts during the treatment. The final data was evaluated on a total of 749 patients.
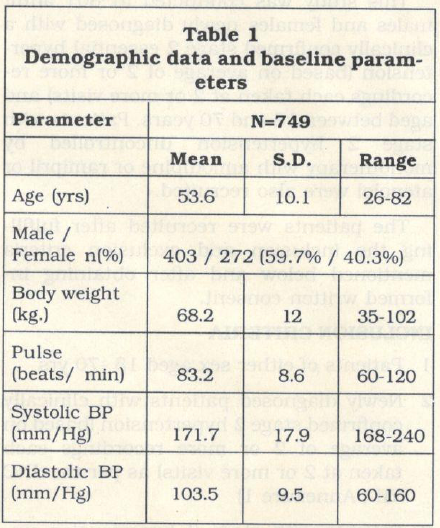 The demographic characteristics of patients are given in Table 1. The mean age of the patients was 53.6 years (range 26-82 years). Of the total patients, 59.7o/o were males and 40.3o/o were females. The mean weight of the patients were 68.2 Kg (range 35-102 Kg).
The demographic characteristics of patients are given in Table 1. The mean age of the patients was 53.6 years (range 26-82 years). Of the total patients, 59.7o/o were males and 40.3o/o were females. The mean weight of the patients were 68.2 Kg (range 35-102 Kg).
The mean heart rate of the patients was per minute). The mean systolic and diastolic blood pressure of the patients was 171.7 mm Hg (range,168- 240 mmHg) and 103.5 mm Hg (range 60-160 mmHg).
The mean systolic blood pressure at base line was 171.9 …± 17.9 mm Hg. This reduced significantly (p < 0.001) to 152.9 ± 16.4 mmHg, 142.1± 13.1 mmHg and 134.3 ± 10.1 mmHg at the end of 1, 2 and 4 weeks of treatment respectively (Table 2, Graph 1).
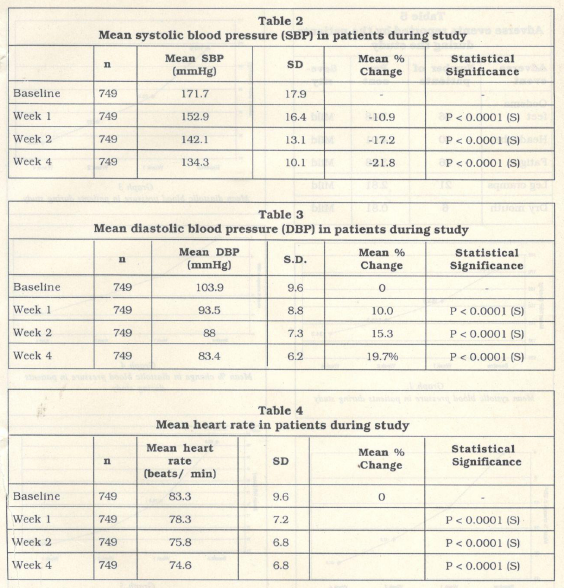

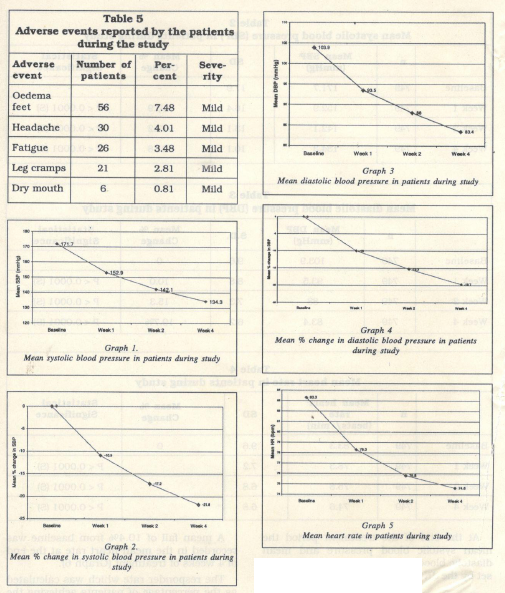
The mean diastolic blood pressure at baseline was 103.9 ± 9.6 mm Hg. This reduced significantly (p < 0.001) to 93.5 ± 8.8 mmHg, 88 ± 7.3 mmHg and 83.4 ± 6.2 mmHg at the end of 1, 2 and 4 weeks of treatment respectively (Table 3., Graph 3).
A mean fall of 19.7o/o from baseline was recorded in the diastolic blood pressure at the end of 4 weeks of treatment (Graph 4).
At the end of the treatment period the mean systolic blood pressure and mean diastolic blood pressure was well below goal set by the JNC VII for hypertension.
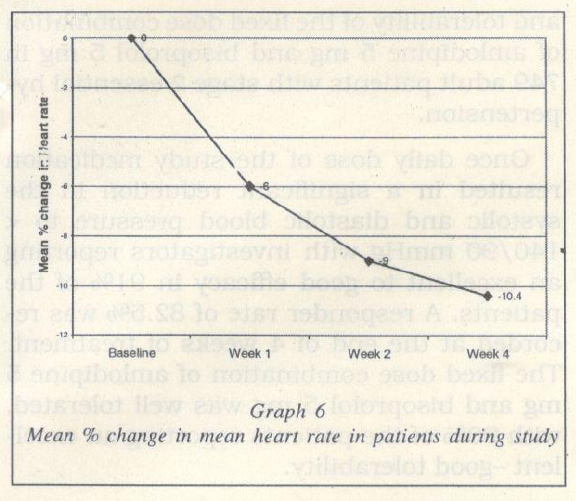
The mean heart rate at baseline was 83.3 ± 9.6 beats per minute. This reduced signifi cantly (p < 0.0001) to 78.3 ± 7.2, 75.8 ± 6.8 and 74.6 ± 6.8 beats per minute, at the end of the 1, 2 and 4 weeks of treatment respec tively (Table 4, Graph 5).
A mean fall of 10.4% from baseline was recorded in the mean heart rate at the end of 4 weeks of treatment (Graph 6).
The responder rate which was calculated as the percentage of patients achieving the blood pressure of< 140/90 mmHg, at the end of 4 weeks of treatment was 82.5.
Adverse events
The most commonly reported adverse event was oedema feet occurring in 8% patients. Other adverse events reported were headache (4%), fatigue (3%), leg cramps (3o/o) and dry mouth (1o/o) (Table 5). All reported adverse events were mild in severity and did not require hospitalization or discontinua tion of therapy.

Efficacy and tolerability

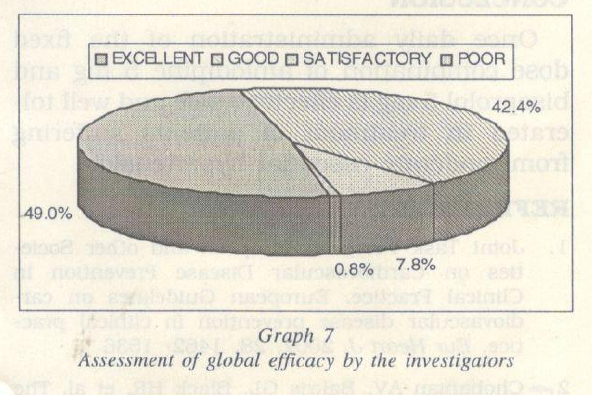
The investigators reported an excellent to good efficacy in 91.4% of the patients (Graph 7). In 7.8% patients the efficacy reported was satisfactory and in 0.8% patients the efficacy reported was poor (Graph 7).


Hypertension affects approximately 50 million individuals in the United States and approximately 1 billion worldwide. As the population ages, the prevalence of hyperten sion will increase even further unless broad and effective preventive measures are implemented. Recent data from the Framingham Heart Study suggest that indi viduals who are normotensive at the age 55 have a 90 per cent lifetime risk for developing hypertension.2
For individuals 40-70 years of age, each increment of 20 mmHg in systolic BP (SBP) or 10 mmHg in diastolic BP (DBP) doubles the risk of CVD across the entire BP range from 115/75 to 185/115 mmHg:2
Reduction in systolic blood pressure to <140 mm Hg and diastolic blood pressure to < 90 mm Hg is associated with a decrease in the incidence of CVD complications.6 Two thirds of hypertensive patients are not being controlled to BP levels less than 140/90 mm Hg. It is therefore recommended that when the blood pressure is more than 20I 10 mmHg above goal, consideration should be given to initiating therapy with 2 drugs, ei them as separate prescriptions or in fixed dose combination.2
It is noteworthy that, only approximately 50% of hypertensive patients respond to monotherapy, and majority of this group (>2/ 3 rd) require the highest recommended dose to achieve control.7
Five major class of anti-hypertensive agents – thiazide diuretics, calcium antago nists, ACE inhibitors, Angiotensin receptor antagonists and beta blockers are suitable for the initiation and maintenance of anti hypertensive treatment, alone or in combi nation. 1
The combination of amlodipine and bisoprolol demonstrates an additive effect since both the drugs have different and com plementary modes of actions to reduce blood pressure: for example, a vascular selective calcium antagonist like amlodipine lowers total peripheral resistance and a beta 1 se lective blocker like bisoprolol lowers heart rate and. thus, cardiac output. Additionally, since both the drugs have to be administered once daily, the rationality of the combination is further strengthened.
In the present study a once daily adminis tration of the fixed dose combination of amlodipine 5 mg and bisoprolol 5 mg demon strated a significant lowering of both systolic and diastolic blood pressure at the end of 4 weeks of treatment.
The mean systolic blood pressure significantly reduced from 171.7 ± 17.9 mm Hg to 134.3 ± 10.1 mmHg by the end of the 41h week of treatment.
A similar decrease was also recorded in the diastolic blood pressure, which reduced from a baseline of 103.9 ± 8.6 mm Hg to 83.4 ± 6.2 mmHg at the end of 4 weeks of treatment.
Both the systolic and diastolic blood pres sures reached the target range as early as 4 and 2 weeks of treatment respectively. The investigators reported an excellent to good efficacy in 91% of the patients.
82.5% of the patients achieved blood pres sure control at values < 140/90 mm Hg at the end of 4 weeks of treatment. This was in compliance with the JNC VII guidelines which mentions that the treatment goal for individuals with hypertension and no other compelling conditions should be < 140/90 mmHg.2
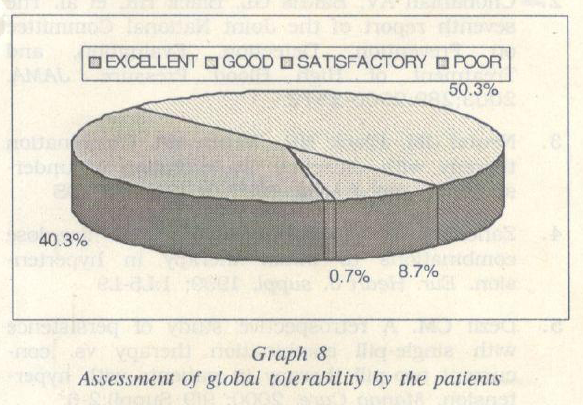
The fixed dose combination of amlodipine 5 mg and bisoprolol 5 mg was generally well tolerated. 90o/o of the patients reported an excellent- good tolerability to the combina tion.
The most commonly reported adverse event was oedema feet occurring in 56 (7.48 o/o) patients. The adverse events reported were mild and did not require hospitaliza tion or treatment.
SUMMARY
The present open, prospective, non com parative study evaluated the safety, efficacy and tolerability of the flxed dose combination of amlodipine 5 mg and bisoprolol 5 mg in 749 adult patients with stage 2 essential hypertension.
Once daily dose of the study medication resulted in a significant reduction in the systolic and diastolic blood pressure to <140/90 mmHg with investigators reporting an excellent to good efficacy in 91% of the patients. A responder rate of 82.5% was re corded at the end of 4 weeks of treatment. The flxed dose combination of amlodipine 5 mg and bisoprolol 5 mg was well tolerated, with 90% of the patients reporting an excel lent -good tolerability.
CONCLUSION
Once daily administration of the fixed dose combination of amlodipine 5 mg and bisoprolol 5 mg is effective, safe and well tol erated in treatment of patients suffering from moderate essential hypertension.
REFERENCES
- Joint Task Force of European and other Socie ties on Cardiovascular Disease Prevention In Clinical Practice. European Guidelines on car diovascular disease prevention In clinical prac tice. Eur Heart J. 2007, 28, 1462- 1536
- Chobanlan AV. Bakris GL, Black HR. et al. The seventh report of the Joint National Committee on Prevention, Detection. Evaluation. and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572
- Neutel JM. Black HR. Weber MA. Combination therapy with diuretics: an evolution of under standing. Am J Med. 1996;101(3A):61S-70S
- Zanchetti A. Contribution of fixed low-dose combinations to Initial therapy Ln hyperten sion. Eur. Heart J. suppl. 1999; 1:L5-L9
- Dezu CM. A retrospective study of persistence with stngle-ptll combination therapy vs. con current two-pill therapy In patients with hyper tension. Manag Care. 2000; 9(9 Suppl):2-6
- Hansson L, et al. Effects of Intensive blood pressure lowertng and low-dose asplrtn Ln pa tients with hypertension: principal results of the Hypertension Optimal Treatment (H011 randomlsed trtal. Lancet 1998; 351: 1755-1762.
- Neutel JM: Low-dose antihypertensive combin-ation therapy: Its rationale and role In cardio-vascular risk management. Am J Hypertens 1999; 12 (8 Pt 2): 73S – 79S.
ACKNOWLEDGEMENT
The authors acknowledge with thanks the following physicians who have contributed in the above study.
Dr. H. Abdul Hamid, (Mysore); Dr. M. Arunachalam, (Madras); Dr. Anna Chhatre, (Pune);Dr. Ashiwini Patki Doshi (Mumbai); Dr. Ashutosh Singh, (Bhopal); Dr. Bhinay Kumar, Gaya; Dr. Bharat Kumrwat, Rattam; Dr. P.K. Bandari, Arunachal, Dr, Behram S.Pardiwala, (Mumbai); Dr. B.S. Bhatia, (Chandigarh); Dr. M.P. Chandrakar; (Durg); D R.J.S. Chabra, (Muzafamagar); Dr. T. Dinakaran, (Madurai); Dr. Deepak Rastogi (Garhwal); Dr. Debashish Choudhary, (Patna); Dr. D.S. Goel, (Ambala); Dr. B. Ghatak, (Assam); D. Gurunath,(Bhilai); Dr. O.K. Das Bhiswas, (Asansol); Dr. S.S. Dhillon, (Durg); Dr. Gunasekar,(Madras); Dr. Ganpat Devpura (Jaipur); Dr. S.R. Gabhir, (Pune); Dr. Hemant Shastri, (Baroda). D.Hariharan. (Chennai); Dr. Keshav Singh, (Chennai); Dr. Krupandhi, (Nellur); Dr. A.K. Kulkarni, (Amaravathi); Dr. B.D. Kkarhade, (Nasik); Dr. Kiran Kumawat (Nasik); Dr. S.K. Kamboj. (Chandigargh) Dr. Krutesh Sha, (Vadodara); Dr. Hussain Khazani, (Hyderabad); Dr. R.C. Kumar, (Baroda); Dr. B.R.J. Kannan, (Madurai); Dr. Lewin Lukose, Quillon; Dr. Maytree Bhanerjee, (Kolkata); Dr. Showkat Mufti (Srinagar); Dr. R. Murali Babu Rao, (Guntur); Dr. Manoj Sharma, (Gaziabad); Dr. P. Manoj Sharma, (Tellichery); Dr. Mehrajudin sha, (Srinagar); Dr. AbdulMajeet. (Srinagar); Dr. A.K. Mahapatra, (Howrah);Dr.R. Manjunath, (Mysore); Dr. Mohammad Ameen, (Vellore);Dr. Meenakshi Sundaram, (Chennai); Dr. Murugesan, (Chennai), D. Manoj Agarwal (Ambhala); Dr. V. Manakavala Perumal, (Tirunelveli). Dr. G. Narender Reddy, (Alwal); Dr. S. Namachivayam,(Kanchipuram); Dr. K. Narasaram (Kumool);Dr. Nitin Sahul, (Indore); Dr. Naina Mohamad, (Madurai); Dr. Praven Kumar, (Vadagara) Dr. Pradeep Mehta, (Indore); Dr. Narayana Swamy Reddy, (Anantapur); Dr. K.K. Pichumani, (T.richy), Dr. S.K. Panda, (Gaya); Dr. R. Pargania, (Raipur), Dr. Naresh Shetty, (Mumbai); Dr. S.K. Patil,(Pune); Dr. H. Prabukar, (Mangalore); Dr. Prakash Mehta, (Vadodara); Dr. Parvaz Hashni, (Bhopal), Dr. Nambiar, (kerala); Dr. Praveen, (Indore); Dr. Pramod Kulkarni, (Dhule); Dr. Parvaiz Shaheed, (Bandhipur); Dr. Paramaswari, (Trichy;) Dr. R.S. Choudari, (Akola); Dr. T.S. Ramaswamy, (Kerala). Dr. Ramash Singhal, (Rewa); D. Rajiv Khera, (Indore); Dr. Jyoti Ranjan Pandey, (Patna); D. F. Jhohar, Mhow, Dr. V. Rajendran, (Trichy;) Dr. Jeffrey, Nagerkqil; Dr. Ramesh Pawar, (Nasik;) Dr. Rakesh Talwar, Chandikar, Dr. K.V. Ramatake, (Chandrapur); Dr. Raja Rathnan, Pondichery; Dr. Jeevaraj, (Madras); Dr. Jahannath Reddy, Karimanagar, Dr. Jaikumar, (Chennai); Dr. A.K. Jain (Dehradun); Dr. Jaipal Chandru, (Sarangpur); D. S. Krishnakumar, Kuzhikode; Dr. V. Jayapal, trtvandrum; Dr. Vuran Rahu; Dr. Atis Basak, (Midnapur); Dr. M.C. Agarwal, (Bhopal); Dr. S.K. Arul. Tirunelveli Dr,. AtulKumar, (Patna); Dr. D. Ambasta.(Dhanbag;) D. Alok Singhal, (Rampur); Dr. Ashok Shari Ali, (Perundurai), Dr. Anil Batra, Bhopal; Dr. Vimal Rai, (Hyderabad); Dr. Vinu Goel. (Mangalore); Dr. Sunil Kumar, (Trivandrum); Dr. Bhatacharya, (Jamshedpur); Dr. Bhatacharya, Dr. A.B.Balsara, (Jamshedp.ur);Dr. R.N. Bhat,(Udupi); Dr.Somath Mitra, (Bangalore); Dr. S.G. Chavan, (Pune);Dr. Shyam Mathur, (Jodhpur); Dr. Sunil Singvi, (Chennai); Dr. Shyam Prasad, (Hyderabad); Dr. Rajpal, Nagerkoil; Dr. Rajesh. Dr. Ravindran, (Chennai); Dr. Sandeep Tak, (Jothpur); Dr. Saxena, (Jaipur) D. Ravi Quillon; Dr. Raj Kapoor, (Moradabad); Dr. Renu, Pratiba Jn; Dr. A.M. Rathor, (Asrinagar); Dr. Raveendra, (Bangalore); Dr. Renu, Pratiba Jn: Dr. A.M. Rathor, (Srinagar); Dr. Raveendra, (Bangalore); Dr. AbdulRahman, (Srinagar); Dr. K. Roy, (Kerala); Dr. B.Ramesh, (Erode); Dr. K.C. Ravi, (Nasik); Dr. Ranganah, (Kumool); Dr. Shabnam Kumar, Kammam; Dr. Satish, (Bangalore); Dr. Hemant (Pune); Dr. Subash Jain (Delhi); D. Sunil Kumar, (Patna); Dr. Srinivas Reddy, (Mehbunagar); Dr. Sushil Jain, (New Delhi). D. Shyam Kumar, (Madurai); Dr. A.K. Pa. Singha, Dr. B.K. P. Singha, (Jamshedpur); Dr. Sankar Das, (West Bengal); D. H.M. Rastogi(Meerut); Dr. Sridhar, Nellore, Dr. Saibhal Guha, (Calicut); Dr. K.V. Sahasranam, (Kerala); D. Shaha; Dr. Sudarshan, (Hydrabad); Dr. Shushil Nandan Sahay, (Jharkhand); Dr. M. (Patna); Dr. Sunil Wadhwa, (Delhi); Dr. Yeli, Devangir; Dr. Vimal Srivastava, Srinivas, (Vishagapatnam); Dr. Showkaf; Dr. Smit, (Raipur). Dr. Shashank; (Surat) Dr. Vivek Dr. G. Shivalingapa, Devangiri; D. Surya Lakshmi, (Vishagatnam); D. Sundar, (Nellore);D. Sunil Kumar, (Patna); Dr. Somasundaram, (Chennai); Dr. Sanjeev,(Delhi); Dr. Vidyasar, Anantpur; Dr. Vivek, Akola); Dr. Geetha Lakshmi, (Bangalore); Dr. D. Suresh (Chennai); Dr. Sushobit Verma, (Bareillei); D. Tiwari, (Bhilai); Dr. A.P. Thiyagarajan, (Madurai); Dr. Tripathy, (Jamshedpur).