India has intensified efforts towards Universal Health Coverage. To achieve this goal, it is important to spend the resources on cost-effective healthcare interventions. Economic evaluations and Health Technology Assessments are important tools to ensure the efficient use of resources. India has made significant progress in this direction, especially with the establishment of Health Technology Assessment (HTA) – India. Further capacity-building and strengthening of HTA is required to ensure the implementation of evidence-based cost-effective interventions in the health sector.
Keywords: Health Technology Assessment, Economic evaluation, Cost-effectiveness
Conflict of Interest: None declared
Source of Support: None declared
Universal Health Coverage (UHC) is one of the most important indicators in the public health arena, which entails ensuring all people have access to quality health services including prevention, promotion, treatment, rehabilitation, and palliation without incurring financial hardship. The concept covers three key elements i.e., access, quality, and financial protection. India is committed to achieving Universal Health care for all by 2030, which is fundamental to achieving the Sustainable Development Goals (Goal 3.8). Out-of-pocket (OOP) expenditure incurred by an average Indian on health was rated as one of the highest (at 67%) in 2014 as per World Health Organization (WHO) data. In 2014, more than 300 million people in India bore the burden of catastrophic spending of 10% or more of their household expenditure on healthcare; besides absolute spending, OOP spending was estimated to push an estimated 85 million people into poverty in the same year. Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (PMJAY) was launched in 2018 as a step towards UHC. It provides insurance coverage to the poorest 40% of the population. Over 50 crore Indians are covered under the scheme with an insurance cover of Rs. 5 lakhs per family. PMJAY provides comprehensive hospitalization coverage for secondary and tertiary care. Its impact can be seen in the latest National Health Accounts Estimates of India 2019-20. As per the report, the share of out-of-pocket expenditure in total health expenditure has declined to 47% in 2019-20. The share of government health expenditure in total health expenditure has increased from 29% in 2014-15 to 41.4% in 2019-20 and per capita government spending on healthcare has doubled. [1]
While the demand for healthcare is practically unlimited, the resources of any country are limited. India with the highest population in the world needs to spend its resources in a way to gain maximum good health for her people. With technological advancements and economic development, the availability of healthcare interventions and the demand for healthcare increases. The policy-makers thus face the difficult task of prioritizing limited resources while investing in interventions for the public healthcare system. The wide spectrum of new interventions will have varied implications on the health outcomes and health budget while taking into consideration equity issues. Economic evaluations offer solutions to these questions to a great extent. Such evaluations can provide evidence-based guidance to the policy-makers to decide on procurement rates of various commodities and the budget impact of investing in cost-effective interventions.
Economic evaluation can be defined as the comparative analysis of alternative courses of action in terms of both their costs and consequences.[2] Any economic evaluation basically measures the costs and outcomes of any given intervention with a comparator. In healthcare, the intervention can encompass different dimensions e.g., diagnosis, treatment, prevention, and program. The comparator can mean either the current best practice or the routine practice or the ‘doing nothing’ scenario. The outcomes can vary across these evaluations. When the outcomes are specific and measured in the form of single natural units, the evaluation is considered a cost-effectiveness analysis. When the outcomes are measured in terms of DALYs (Disability Adjusted Life Years) and QALYs (Quality Adjusted Life Years), they are considered Cost-Utility analysis. It offers more generic measures for various disease outcomes. Cost-benefit analysis is a type of economic evaluation where both the costs and outcomes are measured and compared in monetary terms. The results are in the form of incremental cost-effectiveness ratio, cost-utility ratio, or cost-benefit ratio respectively, which basically show the additional number of resources required for gain of one additional unit of gain in outcome.
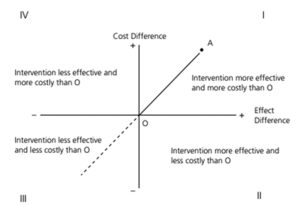
The analysis can thus result in one of the four scenarios depicted in Figure 1.
The interventions falling in zone II and IV are easy to analyse, but when the intervention falls in zone I and III, careful analysis of the result is required. Here the concept of Cost-Effectiveness Threshold (CET) is useful. CET works as a benchmark beyond which the additional cost will not be considered cost-effective. Researchers have suggested the need of India specific CET.[3] In its absence, Indian researchers use one times GDP per capita as the threshold (HTA Reference Manual DHR). But India’s status in terms of UHC, availability of resources and the disease burden is peculiar. Thus, country specific CET can help in generating better quality evidence through economic evaluations. CET is also important for sensitivity analysis which can suggest at what cost the intervention will be cost-effective. Such an analysis plays an important role in price negotiations.
Economic evaluation with considerations of ethical and equity issues is known as Heath Technology Assessment (HTA). HTA is a systematic and multidisciplinary evaluation of the properties of health technologies and interventions covering both their direct and indirect consequences. As per European Network for Health Technology Assessment, HTA is a multidisciplinary process that summarizes information about the medical, social, economic, and ethical issues related to the use of health technology in a systematic, transparent, unbiased, and robust manner.[4]
Evidence generated through HTA can help in standard treatment guidelines. It can help in deciding the reimbursement package under public health insurance schemes. In its absence, they are decided based on expert opinion, but it’s very subjective and is not backed by robust evidence. Thus, HTA can help in increasing evidence-based policy-making.
Global and Indian Scenarios
In the late 1970s, methods of HTA were established in the USA by the U.S. Office of Technology Assessment. In the late 1980s, Sweden and Japan established institutions for HTA. Gradually other European countries, Canada, Latin American countries, and Asia have created institutions for HTA in their respective countries. The institutionalization of HTA has been slower in Asia, with the first HTA Agency in Malaysia in 1995. This is mainly because of a lack of awareness, country-specific epidemiological, clinical, and health economics data, and a lack of collaborative research approach.[5] In 2014, World Health Assembly passed a resolution to use HTA for UHC. This has given a global emphasis to HTA.
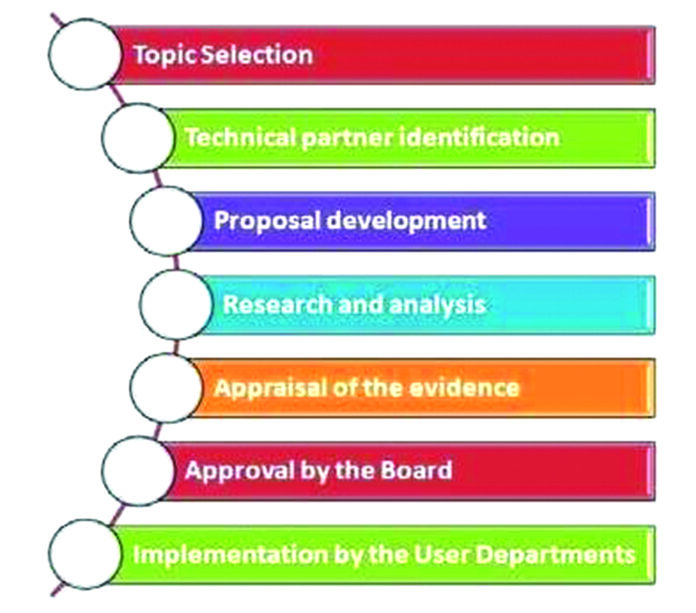
In India, the high-level expert group on UHC report in 2011 gave a great impetus to HTA. Twelfth five-year plan and National Health Policy, 2017 emphasized on the importance of HTA in introducing new technologies into public health programmes. The National Health Policy, 2017 also recommended an institutional framework for HTA on the lines of UK’s National Institute for Clinical and Care Excellence (NICE). In line with this, Medical Technology Assessment Board was planned in India. In 2017, Health Technology Assessment India (HTAIn) was established in India with secretariat in Department of Health Research (DHR) under Ministry of Health and Family Welfare. It is designed as a hub and spoke model where several institutions are identified as Regional Resource Centres and Technical Partners. There is an overarching HTA Board which takes final policy decisions based on recommendations of HTA studies.[6] HTAIn has uploaded a manual on its website, which can be useful for those involved in HTA studies and its users.[7]
There have been several studies undertaken by HTA Resource Hubs on questions proposed by the user departments from the Ministry be it local, state or centre. Many of the outcomes have resulted in introducing cost-effective technologies in the program settings. Based on the findings of a large multicentric study in India on estimating the Cost of Health Services in India, PMJAY benefits packages were revised in 2019.
A systemic review of all the published economic evaluations related to India between 1980 and 2014 highlighted the need of more such studies in healthcare sector in India with improved quality. The researchers also emphasized upon the need of capacity-building of researchers for this. The need for region- or state-specific studies that account for variations in epidemiological transition, health-care costs, and health-care infrastructure across the country were few of the points for action[8]. Based on these, the instillation of HTAIn Board by the Ministry within Department of Health Research and capacity building on conduct of HTA studies by academic research bodies across India has made a great headway.
Conclusion
HTA is an additional arm to the decision-making process apart from clinical effectiveness of interventions. It can help both government and private health sectors in making rational and evidence-based decisions to ensure effective health interventions are affordable, accessible to all including the most vulnerable such that India achieves the UHC target that it has committed towards SDG 2030.
For more details, please refer to https://htain.icmr.org.in/
References
- National Health Accounts-2019-20.pdf [Internet]. [cited 2023 Jul 4]. Available from: https://nhsrcindia.org/sites/default/files/2023-04/National%20Health%20Accounts-2019-20.pdf
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Prinja S, Sundararaman T, Muraleedharan VR. Cost-effectiveness Threshold and Health Opportunity Cost. 2020;(2).
- Technology (U.S.) NIC on HSR& HC. HTA 101: Introduction to Health Technology Assessment [Internet]. U.S. National Library of Medicine; [cited 2023 Jun 23]. Available from: https://www.nlm.nih.gov/nichsr/hta101/ta10103.html
- Liu G, Wu EQ, Ahn J, Kamae I, Xie J, Yang H. The Development of Health Technology Assessment in Asia: Current Status and Future Trends. Value Health Reg Issues. 2020 May 1; 21:39–44.
- Health Technology Assessment in India (HTAIn) – About HTAIn [Internet]. [cited 2023 Jun 28]. Available from: https://htain.icmr.org.in/about-us/about-htain
- htain manual.pdf [Internet]. [cited 2023 Jul 4]. Available from: https://htain.icmr.org.in/images/pdf/htain%20manual.pdf
- Prinja S, Chauhan AS, Angell B, Gupta I, Jan S. A Systematic Review of the State of Economic Evaluation for Health Care in India. Appl Health Econ Health Policy. 2015 Dec;13(6):595–613.