Abstract
Asthma research has advanced much through recent insights into its pathophysiology and cellular interactions. Despite implementing the National Asthma Education and Prevention Program (NAEPP) and Global Initiative for Asthma (GINA) guidelines, 10% of asthma remains refractory to conventional therapy. Asthma is now subclassified into different phenotypes that have distinct immunological features. Eosinophilic asthma is a well-known phenotype of severe asthma; however, a large body of clinical and experimental evidence strongly associates persistent airway inflammation, including the accumulation of neutrophils in the bronchial mucosa, and resistance to corticosteroid therapy. This is identified as non-Type-2 immune responses leading to severe asthma and poor response to steroid therapy. Importantly, mainstay therapies are often ineffective in severe asthma, and effective alternatives are urgently needed.
Keywords: IgE, IL-4, IL-5, IL-13, GATA3, Thymic stromal lymphopoietin (TSLP), Th2
Introduction
Asthma has been conventionally considered a Th2-mediated inflammatory disorder that requires an anti-inflammatory controller therapy. However, it has been demonstrated that there are subpopulations of patients in whom standard therapy fails. Many studies proposed a paradigm shift in the characterization of asthma subgroups that may differentially respond to novel therapies.1,2,3,4 The development of biological formulations targeting specific molecular pathways is a result of an improved understanding of the genetic and molecular mechanisms of asthma pathogenesis over the past few decades.2
World Asthma Day is observed on May 6, as a yearly reminder that asthma deserves more attention, management, and evolution in therapies.
Molecular Phenotypes and Targeted Therapies
Within the type-2 asthma group, there appears to be heterogeneity, including patients with late-onset eosinophilic disease that respond to anti-IL-5 therapy.3 Importantly, targeting one or more cytokines within a particular asthma endotype can potentially improve clinical outcomes in patients with refractory disease. IL-4 and IL-13 have distinct and overlapping roles in asthma pathophysiology. Both cytokines signal through the type 2 IL-4 receptor (IL-4Rα and IL-13Rα1 heterodimer) and are regulated by the master TH2 transcription factor GATA binding protein 3. IL-4 induces IgE class switching of B cells and is critical for Th2 cell differentiation, whereas IL-13 promotes cellular influx, airway hyperresponsiveness, and remodeling features.2,4,5
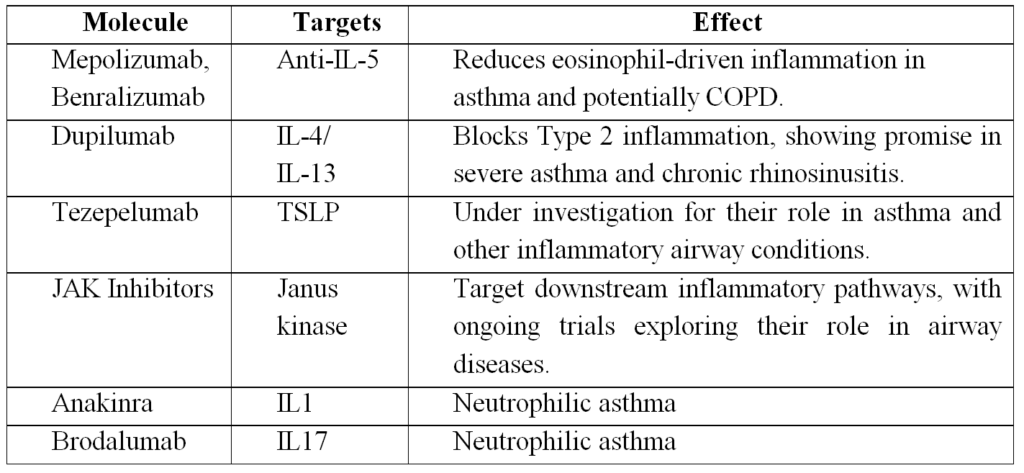
Based on the various roles of IL-4 in Th2-mediated asthma, initial efforts focused on targeting IL-4 for therapeutic intervention (Table 1). However, early studies with anti-IL-4 treatment failed to demonstrate clinical efficacy, possibly due to IL-4/IL-13 redundancy and lack of subject phenotyping.2 Thus, a strategy to block both IL-4 and IL-13 signalling in patients with elevated biomarkers of type 2 inflammation was pursued.2,6 The humanized monoclonal antibody dupilumab binds to the IL-4R α chain (shared receptor subunit for IL-4 and IL-13) and was evaluated in a prospective study of moderate to severe asthmatics with a type 2-high phenotype (sputum or blood eosinophilia >= 3% or 300 cells/uL, respectively). Importantly, the treatment group exhibited significant reductions in asthma exacerbations, as well as improvements in other clinical outcomes, including quality of life and lung function (but not blood eosinophilia), highlighting the importance of targeting a specific asthma endotype with selective therapy.6
Further studies with anti-IL-13 treatment also showcased the impact of molecular phenotypes on treatment success. A double-blind placebo-controlled trial of 219 uncontrolled adult asthmatics investigated the efficacy of lebrikizumab in all subjects as well as in the type 2-high subgroup defined by elevated serum periostin levels.7 Although the total cohort demonstrated improved FEV1 in subjects receiving lebrikizumab compared to placebo, there were no differences in other measured clinical outcomes. However, patients who possessed high serum periostin levels pre-treatment had more significant improvements in FEV1 as well as asthma exacerbations compared with placebo.
IL-5 is a pro-eosinophilic type 2 cytokine that binds to the IL-5 receptor α expressed on eosinophils and basophils and promotes eosinophil recruitment, survival, and activation. Unfortunately, early studies evaluating the humanized anti-IL-5 monoclonal antibody mepolizumab in non-phenotyped mild intermittent and moderate persistent asthma subjects also lacked favorable clinical outcomes despite reduced eosinophil counts.2 The lack of early success of anti-IL-5 treatment may have been due to the enrolment of heterogeneous populations of asthmatics, and subsequent investigations were able to demonstrate the efficacy of anti-IL-5 treatment in patients with increased eosinophils. In asthmatics with elevated blood eosinophil levels and marginal asthma control despite treatment with high-dose inhaled or oral glucocorticoids, asthma exacerbations were reduced in those receiving mepolizumab compared with placebo. The treatment group also had improved FEV1, quality of life, and ACQ scores.8
Reslizumab is another humanized anti-IL-5 monoclonal antibody in Phase 3 trials that has demonstrated significant reductions in asthma exacerbations in patients with inadequately controlled asthma and elevated blood eosinophil levels.9 Benralizumab, a humanized monoclonal antibody that targets IL-5 receptor α, has been shown in a phase 2b dose-ranging trial to decrease asthma exacerbations in severe eosinophilic asthma compared to placebo at higher doses.10 Importantly, one dose of benralizumab has been shown to reduce asthma exacerbations in severe asthmatics presenting to the ED by 49% and hospitalizations associated with these exacerbations by 60% compared to a placebo in a 12-week follow-up period.11 In summary, targeting eosinophil-high patients appears to hold the most promise for the anti-IL-5/IL-5R treatment strategy.
Newer targets
A very recent proof-of-concept trial evaluated the GATA3-specific DNA enzyme SB010 on early and late-phase allergen challenge responses in mild allergic asthmatics.12 SB010 cleaves GATA3 mRNA transcripts that in turn reduces the downstream induction of GATA3-regulated genes including IL-4, IL-5 and IL-13. The study showed that early and late-phase responses were attenuated by 11% and 34%, respectively, after 28 days of inhaled SB010. Whether this strategy will have efficacy in severe asthma is currently unknown.
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine that induces activation of innate and adaptive type 2 responses in human asthmatic airways that have been shown to have higher levels of TSLP compared to healthy controls.2 A recent double-blind placebo-controlled study investigated the effect of AMG 157, a fully human anti-TSLP monoclonal antibody, on early and late asthmatic responses after an allergen challenge.13 The treatment groups showed a decrease in early and late-phase FEV1 reduction as well as blood and sputum eosinophil counts and FENO. Though this study was a small proof-of-concept trial, targeting cytokines upstream of several effector cells in type 2 immunity may hold promise as a future therapeutic strategy.
The long-acting muscarinic receptor antagonist tiotropium is not currently FDA-approved for asthma, although recent clinical data is promising. A systematic review of tiotropium revealed a reduction in exacerbations as well as lung function improvements in severe asthmatics not optimally controlled on ICS/LABA.14
Type 2-low (Th2 low) asthmatics do not have unifying biomarkers but typically have later onset disease associated with neutrophilia, smoking, obesity, and infection.2 Unfortunately, recent strategies to target neutrophilic asthma have not yielded the same success as the novel therapies for Th2-high asthma.
The TNF-α receptor inhibitor etanercept demonstrated some improvement in AHR, lung function, and symptoms in a small number of severe corticosteroid-dependent asthmatics.2 The monoclonal antibody to TNF-α, infliximab, reduced asthma exacerbations in moderate asthmatics, but a subsequent double-blind placebo-controlled study using another humanized antibody to TNF-α, golimumab, did not demonstrate any improvement in FEV1 or reduction in asthma exacerbations. Importantly, an increase in systemic infections and malignancies led to the early termination of the trial.2
Neutrophilic asthma has been linked to higher ICS use and lower FEV1. CXCR2 or C-X-C motif chemokine receptor 2, is the receptor for IL-8 that mediates neutrophil migration to sites of inflammation. An antagonist of CXCR2 receptor was developed to target neutrophilic asthma, but human studies did not demonstrate clinical benefit.2 More recently, anakinra, an IL-1 receptor antagonist, was shown to decrease airway neutrophilia in a proof-of-concept study of healthy subjects challenged with inhaled LPS and maybe a future therapeutic strategy in those with neutrophilic asthma.15
Lastly, brodalumab is an anti-IL-17 receptor antibody targeting the Th17 pathway that has roles in host defence and tissue neutrophilia.2 Unfortunately, brodalumab treatment in severe asthmatics did not show an improvement in clinical benefit compared to placebo even when multiple subset analysis was performed concerning ACQ (Asthma Control Questionnaire) score, FEV1, and symptoms.2
Conclusion
The future of airway disease management is shifting toward a more individualized, biomarker-driven approach that combines cutting-edge biologics, small molecules, gene therapies, and advanced drug delivery systems. Leveraging novel technologies and a deeper understanding of airway pathophysiology will enable clinicians to achieve better disease control and improved quality of life for patients with chronic airway diseases.
References
- Wenzel ME, Busse WW, National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007; 119: 14–21.
- Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015; 135:299–310.
- Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol. 2015; 308: L130 40.
- Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–60.
- Wills-Karp M, Finkelman FD. Untangling the Complex Web of IL-4– and IL–13–Mediated Signalling Pathways. Sci Signal. 2008;1(51):pe55.
- Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–66.
- Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–98.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–207.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomized placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–66.
- Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879–90
- Krug N, Hohfeld JM, Kirsten A, et al. Allergen-Induced Asthmatic Responses Modified by a GATA3-Specific DNAzyme. N Engl J Med. 2015; 372:1987–1995.
- Nowak RM, Parker JM, Silverman RA, et al. A randomized trial of benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015; 33:14–20.
- Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014; 370:2102–10.
- Rodrigo GJ, Castro-Rodríguez JA. What Is the Role of Tiotropium in Asthma? A Systematic Review with Meta-analysis. Chest. 2015; 147:388–96.
- Hernandez ML, Mills K, Almond M, et al. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol. 2015; 135:379–85.