A petition urging the immediate suspension of all COVID-19 mRNA vaccines has been signed by numerous doctors and healthcare professionals worldwide, citing concerns about their potential link to a rise in excess deaths. The petition, known as the HOPE Accord, was launched recently to raise awareness about the risks associated with the continued use of gene-based mRNA vaccines, which were originally granted emergency use authorization during the COVID-19 pandemic.
“A growing body of evidence suggests that the widespread rollout of the novel COVID-19 mRNA vaccine products is contributing to an alarming rise in disability and excess deaths,” the HOPE Accord states.
It further argues that the emergency situation under which these vaccines were authorized no longer exists, and the burden of proof now lies on those advocating for their continued use to demonstrate they are not causing harm. “Until such evidence is presented, regulators should suspend their use as a matter of standard medical precaution,” the petition reads.
Accompanying the petition, British Indian Consultant Cardiologist Dr. Aseem Malhotra has written an open letter to the General Medical Council (GMC) in the UK. Dr. Malhotra, a vocal advocate for evidence-based medical practices, highlighted serious concerns about vaccine safety, citing clinical trials, observational data, pharmacovigilance reports, and autopsy findings.
“Serious harms from the vaccine have been confirmed, including deaths within two weeks of administration in many cases,” he wrote. “The data is clear that, for some individuals, the risks of the vaccines outweigh the benefits.”
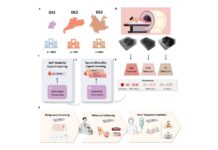
The petition also calls for fully resourced independent investigations to reevaluate the safety and effectiveness of all COVID-19 vaccine products. It demands a detailed exploration of the mechanisms of harm, alongside a reassessment of vaccine effectiveness based on real-world clinical outcomes rather than modeled data.
Additionally, the petition emphasizes the need for transparency from government bodies and pharmaceutical companies, calling for access to anonymized patient-level data from clinical trials and surveillance programs. It also encourages the scientific community to publish findings from any unpublished COVID-19 vaccine studies to address potential publication bias.
“These cumulative actions will help determine the true extent of harm caused by these products and their actual benefit in real-world scenarios,” the petition concludes.
As reported by economictimes, the debate around COVID-19 vaccines continues to fuel global discussions on public health policies, highlighting the importance of transparency and evidence-based approaches to ensure the safety and well-being of populations.