
An international team of scientists has discovered that cholesterol can accumulate inside the mitochondria of heart muscle cells, or cardiomyocytes, severely disrupting their function. For the first time, researchers have identified the exact cellular mechanism by which cholesteryl esters—carried by lipoproteins—enter heart cells and impair their energy-producing structures.
The findings, recently published in the Journal of Lipid Research, were led by Dr. Vicenta Llorente-Cortés of the Institute of Biomedical Research of Barcelona (IIBB-CSIC), with contributions from institutions in Spain, the U.S., and France.
Why the Heart Is Especially Vulnerable to Lipid Damage
The heart relies heavily on mitochondrial function to maintain its constant, high-energy contractions. In fact, mitochondria make up nearly one-third of a cardiomyocyte’s volume. These organelles use oxidative phosphorylation to convert nutrients into ATP—the molecule that powers heart function.
However, in conditions like obesity, diabetes, and hypercholesterolemia, mitochondrial function often deteriorates, leading to a decline in heart performance. This study pinpoints how lipoprotein-bound cholesteryl esters infiltrate heart cells and accumulate in mitochondria, resulting in structural and functional damage.
LRP1: The Gateway for Cholesterol Entry into Mitochondria
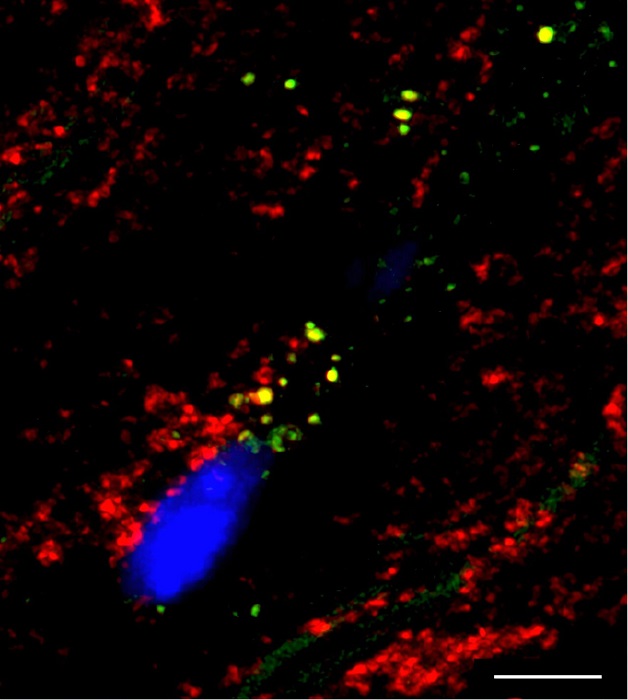
The research team identified the LRP1 receptor—located on the surface of cardiomyocytes—as the key transporter responsible for moving esterified cholesterol into cells. Under lipotoxic conditions, LRP1 facilitates the entry of cholesteryl esters into mitochondria, where they compromise mitochondrial membranes, disrupt the respiratory chain, and hinder energy production.
“This mechanism shows that cholesterol doesn’t just pose a threat through plaque formation in arteries,” said Dr. Llorente-Cortés. “It also invades heart cells and sabotages their internal energy systems.”
A Targeted Immunotherapy Reverses Mitochondrial Damage
To counteract this harmful process, the team developed an experimental immunotherapy using monoclonal antibodies that block the P3 domain of the LRP1 receptor. This highly targeted approach prevents the receptor from transporting cholesteryl esters into the cell.
In trials using a rabbit model that closely mimics human lipid metabolism, the therapy significantly reduced mitochondrial cholesterol buildup. It also restored mitochondrial structure, including the regeneration of cristae—folds essential for efficient energy production.
Restored Energy Production and Metabolic Function
Following treatment with anti-P3 antibodies, researchers observed a clear improvement in mitochondrial function. Oxidative phosphorylation efficiency increased, ATP production normalized, and overall energy output of cardiomyocytes improved.
Moreover, the therapy enhanced the interaction between mitochondria and cytoplasmic lipid droplets, signaling a broader reorganization of cellular metabolism. These changes not only halted cholesterol-induced damage but actively reversed mitochondrial dysfunction.
Multidisciplinary Tools Reveal Hidden Cellular Pathways
The researchers combined cutting-edge techniques—bioenergetics analysis from the University of California, mass spectrometry from the University of Toulouse, and advanced imaging from IR Sant Pau and the University of Barcelona—to unravel the mechanism. Using a dyslipidemic rabbit model, they isolated mitochondria, quantified lipid accumulation, and assessed respiratory chain performance under varying cholesterol levels.
Implications for Treating a Range of Heart Conditions
As reported by medicalxpress, this therapeutic strategy holds promise for treating cardiovascular conditions driven by abnormal lipid profiles, including obesity, chronic hypercholesterolemia, and myocardial ischemia. By intervening at the intracellular level—specifically inside mitochondria—the approach offers a novel way to protect the heart from metabolic deterioration.
“Our treatment reaches where others don’t—deep inside the mitochondria, where the heart’s energy is generated,” emphasized Dr. Llorente-Cortés.
Addressing a Critical Clinical Gap
Cardiovascular diseases cause one in three deaths globally. While existing treatments manage external risk factors like high blood pressure and cholesterol, they fall short of addressing internal cellular damage. This research provides a pioneering solution by targeting cholesterol accumulation within the heart’s energy centers.
“Lowering cholesterol in the blood is important, but it’s no longer enough,” concluded Dr. Llorente-Cortés. “We must protect the heart from within—by preserving its mitochondria and the energy they provide.”