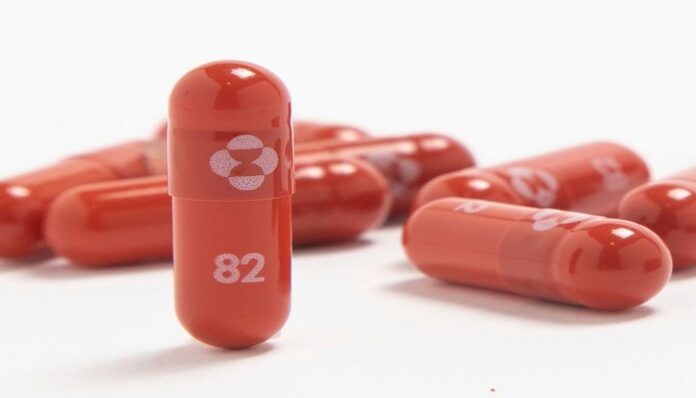
After CDSCO’s approval for emergency use of Covid antiviral pill Molnupiravir, as many as thirteen Indian companies can now manufacture and market the drug. Molnupiravir, developed by Merck and Ridgeback Biotherapeutics has already been approved by the UK and the US Food and Drug Administration, recommending it for adult patients who have at least one risk factor for developing severe illness.
The drug inhibits replication of the Sars-CoV-2 virus by targeting the part of the virus called ribonucleic acid polymerase, which has not changed much even after mutations in the Omicron variant.
The recommended dosage is 800 mg twice a day for 5-days for treatment of adult patients with SpO2 over 93 per cent and who have high risk of progression of the disease, including hospitalisation or death. As per CDSCO, the drug should only be sold under the prescription of medical specialists.
Soon after, companies like Torrent Pharma, Sun Pharma, Cipla, Hetero, Dr Reddy’s Laboratories (DRL) rushed in to announce that they would soon launch the drug in India.
Sun Pharma will launch the drug under the brand name Molxvir and should be available in a week. Likewise, Torrent pharma will launch the drug under the brand name Molnutor, Hetero will launch it as Movfor — a 40 capsule pack (200 mg) that will be marketed by its associate company Hetero Healthcare, Dr. Reddy’s will soon launch its Molnupiravir capsules 200 mg under the brand name Molfluand Cipla will brand it as Cipmolnu.
These five companies had already individually entered into non-exclusive voluntary licensing agreements with Merck Sharp Dohme (MSD) to manufacture and supply molnupiravir.