SK Biopharmaceuticals, a leading South Korean biotech company, has signed a collaboration agreement with the Korea Institute of Radiological and Medical Sciences (KIRAMS), the nation’s premier research institute in radiological and medical sciences. Together, they aim to advance preclinical radiopharmaceutical drug discovery and development.
As reported by biospectrumasia, this partnership marks a significant milestone, as both parties will focus on developing radiopharmaceutical compounds and exploring innovative cancer treatments utilizing actinium-225 (225Ac), an alpha-particle-emitting radioisotope known for its ability to selectively destroy cancer cells.
Radiopharmaceutical therapy (RPT), a cutting-edge area of nuclear medicine, has garnered significant attention for its potential to revolutionize oncology. SK Biopharmaceuticals has already begun 225Ac-based research, sourcing the isotope from TerraPower Isotopes, a subsidiary of TerraPower—a nuclear innovation company backed by SK Biopharmaceuticals’ parent company, SK Inc., and Bill Gates.
Under the agreement, SK Biopharmaceuticals and KIRAMS plan to submit an Investigational New Drug (IND) application by 2027. By leveraging KIRAMS’ expertise, facilities, and equipment, the partnership aims to reduce the time and costs involved in drug development while accelerating the expansion of SK Biopharmaceuticals’ RPT pipeline.
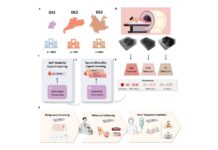
This initiative aligns with SK Biopharmaceuticals’ recently announced “RPT Roadmap,” which sets a vision to become a global leader in radiopharmaceutical therapies by 2027. The roadmap includes discovering new compounds, expanding supply and production capacities, and developing advanced technology platforms through strategic collaborations and internal innovation.
As part of its roadmap, SK Biopharmaceuticals has also secured a supply agreement for 225Ac and in-licensed SKL35501 (FL-091), a radiopharmaceutical compound targeting neurotensin receptor 1 (NTSR1) solid tumors. These efforts underscore the company’s commitment to strengthening its RPT capabilities and advancing innovative cancer treatments.