
Researchers at Vanderbilt University School of Medicine Basic Sciences have identified the first known example of activity-dependent development in hypothalamic neural circuits.
While previous studies have established that the hormone leptin directly acts on hunger neurons via leptin receptors to promote neural circuit development, new findings published in PNAS on November 25 reveal that certain neurons without leptin receptors are still influenced by leptin’s activity.
As reported by medicalxpress, led by Dr. Richard Simerly, Louise B. McGavock Professor and a faculty member in molecular physiology and biophysics, the research uncovers a novel role for leptin in shaping neural circuits related to autonomic regulation and food intake. The team demonstrated that silencing the activity of hunger neurons, known as AgRP neurons, during a critical postnatal period can have lasting structural and functional impacts on circuits responsible for energy balance.
Leptin, a hormone primarily known for regulating hunger and body weight in adults, also plays a crucial role in forming circuits that control homeostasis during early development.
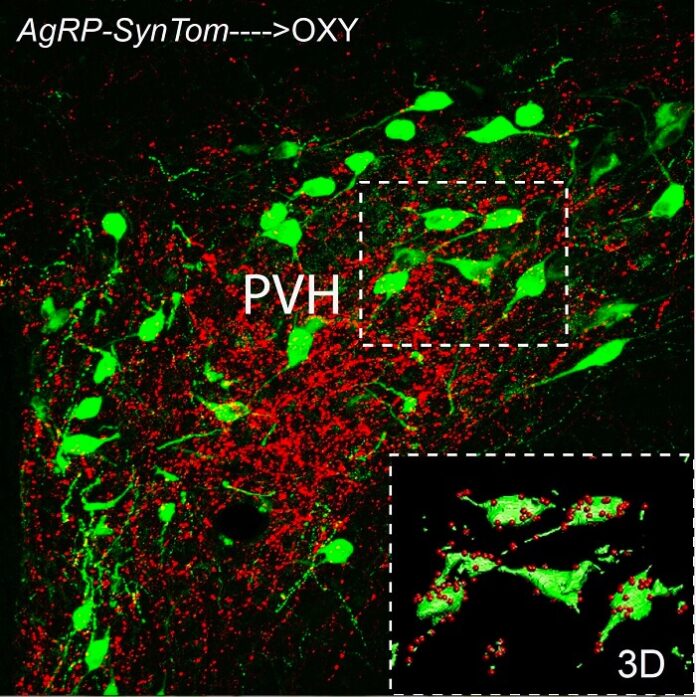
The study used advanced techniques such as light-sheet fluorescence microscopy and 3D imaging to observe oxytocin neurons within the hypothalamic paraventricular nucleus (PVH), uncovering new insights into the interactions between leptin-sensitive and non-leptin-sensitive neurons.
Key Findings:
- Role of Leptin in Neural Circuit Development:
Leptin is essential for forming normal neural connections between hypothalamic oxytocin neurons and brainstem neurons, which regulate autonomic responses related to feeding, even though oxytocin neurons in this pathway lack leptin receptors. - Activity-Dependent Development of Neural Circuits:
The development of circuits connecting the hypothalamus and brainstem relies on the activity of leptin-sensitive AgRP neurons during a critical postnatal window. - Long-Term Effects of Neural Activity Perturbation:
Disrupting neural activity in hypothalamic neurons during development can permanently alter the brainstem’s regulation of gastrointestinal processes associated with feeding.
These findings underscore the broader role of leptin and neural activity in organizing essential metabolic circuits, with implications for understanding metabolic health and disease risk.
Dr. Simerly highlighted the significance of this research, stating, “The discovery that neural circuits controlling energy balance are sensitive to activity during critical developmental periods suggests that many factors could influence hypothalamic development through this mechanism.”
He added that exposure to molecules altering neural activity in early brain development may have lasting effects on neural circuits, potentially impacting brain function in both health and disease.
The study also opens new avenues for leveraging this mechanism to support normal brain development and improve outcomes for individuals at risk due to genetic or environmental factors.
The research was made possible by the contributions of staff scientist and first author Jessica Biddinger, as well as collaborating author Julio Ayala, associate professor of molecular physiology and biophysics and director of the Vanderbilt Mouse Metabolic Phenotyping Center.






















