
Revolutionizing Late-Stage Cancer Treatment
Patients with late-stage cancer often endure multiple rounds of treatment, which can cause severe side effects and may not always be effective. In an effort to expand treatment options, MIT researchers have developed tiny implantable particles that deliver both heat and chemotherapy directly at the tumor site.
Targeted Treatment with Fewer Side Effects
Unlike traditional intravenous chemotherapy, which affects the entire body, this localized approach minimizes side effects while maximizing treatment efficacy. By combining phototherapy and chemotherapy, this dual-therapy method extends patient survival more effectively than administering each treatment separately. In a study with mice, the therapy eliminated tumors in most cases and significantly prolonged survival rates.
A Potential Game-Changer for Aggressive Tumors
“This technology could help control the growth of aggressive tumors in patients with limited treatment options,” explains Ana Jaklenec, a principal investigator at MIT’s Koch Institute for Integrative Cancer Research. “The goal is to prolong life and improve quality of life during treatment.”
Jaklenec co-authored the study alongside Angela Belcher, James Mason Crafts Professor of Biological Engineering and Materials Science and Engineering, and Robert Langer, an MIT Institute Professor. Lead author Maria Kanelli, a former MIT postdoc, published the findings in ACS Nano.
Innovative Dual Therapy Approach
Patients with advanced tumors typically undergo a mix of chemotherapy, surgery, and radiation. MIT’s new approach integrates phototherapy, a method that heats implanted particles with an external laser to destroy tumor cells.
Previously, gold nanoparticles were used for phototherapy in clinical trials, but MIT’s team opted for molybdenum disulfide, a highly efficient material for converting laser light into heat. This allows the use of lower-powered lasers, reducing potential damage to surrounding tissues.
Creating Smart Microparticles for Therapy
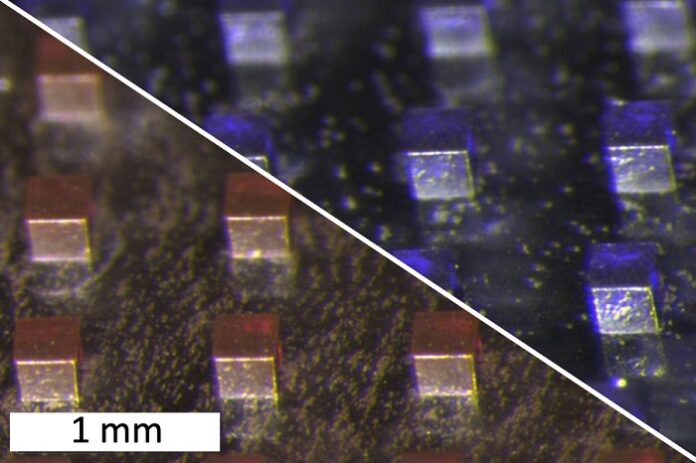
To develop the microparticles, researchers combined molybdenum disulfide nanosheets with chemotherapy drugs—either doxorubicin (hydrophilic) or violacein (hydrophobic). They mixed these components with a polymer, polycaprolactone, then dried the mixture into a film. This film was later compressed into 200-micrometer cubic particles, which remain in the tumor site during treatment.
Each treatment cycle involves using a near-infrared laser to heat the particles. This laser penetrates a few millimeters to centimeters into the tissue, ensuring a localized effect.
“The advantage of this platform is its ability to be activated on demand,” says Kanelli. “After a single intratumoral injection, an external laser triggers the drug release while simultaneously applying thermal therapy.”
Machine Learning Optimizes Treatment
As reported by news.mit.edu, the researchers used machine-learning algorithms to determine the ideal laser power, irradiation time, and phototherapeutic concentration for optimal outcomes. Their optimized treatment cycle lasts approximately three minutes, heating the particles to 50°C—sufficient to kill tumor cells while releasing chemotherapy drugs from the polymer matrix.
“This machine-learning-optimized laser system enables precise, localized chemotherapy with minimal systemic toxicity compared to traditional regimens,” explains Neelkanth Bardhan, a Break Through Cancer research scientist and second author of the study.
Eliminating Tumors in Mice
The team tested their treatment on mice with aggressive triple-negative breast cancer. After implanting about 25 microparticles per tumor, they performed three laser treatments, spaced three days apart.
“This demonstration highlights the power of near-infrared-responsive materials,” says Belcher. “Releasing chemotherapy at timed intervals using light after a single dose of particles makes treatments less painful and improves patient compliance.”
Mice receiving this therapy showed complete tumor eradication and significantly extended lifespans compared to those receiving only chemotherapy, phototherapy, or no treatment. The polymer used in the microparticles is FDA-approved for medical devices, increasing the potential for human clinical trials.
Next Steps Toward Human Trials
MIT researchers now plan to test the microparticles in larger animal models before advancing to clinical trials. They believe this technology could be effective for a wide range of solid tumors, including metastatic cancers.
This research was funded by the Bodossaki Foundation, the Onassis Foundation, a Mazumdar-Shaw International Oncology Fellowship, a National Cancer Institute Fellowship, and the Koch Institute Support Grant from the National Cancer Institute.