
Researchers at the Indian Institute of Science (IISc) have uncovered new insights into how cancer cells adapt their movement based on their surroundings, offering potential pathways for understanding and controlling metastasis.
Published in the Biophysical Journal, the study demonstrates how variations in cancer cell properties and their interactions with the microenvironment influence their migratory behavior. The findings shed light on why certain cancer cells spread more aggressively in specific conditions, particularly in ovarian cancer.
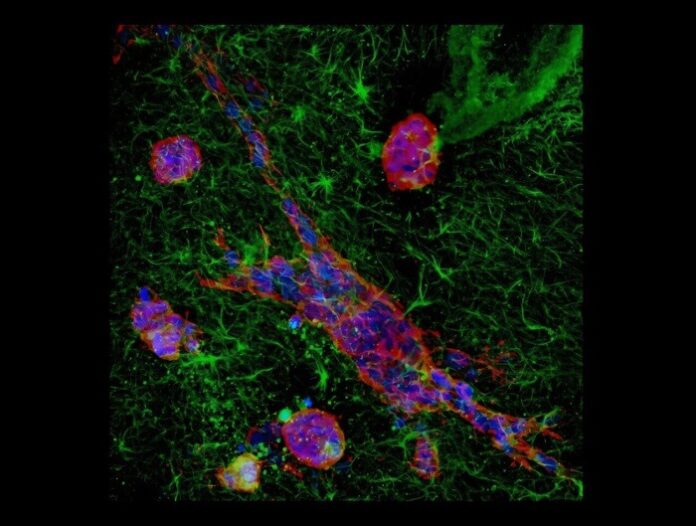
As reported by medicalxpress, the team investigated two types of ovarian cancer cells—OVCAR-3, with a polygonal shape, and SK-OV-3, with a spindle-like structure. By placing these cells on soft and stiff surfaces that mimic healthy and diseased tissues, the researchers observed distinct migration patterns.
On softer surfaces, akin to healthy tissue, both cell types moved slowly and randomly. However, on stiffer surfaces that mimic scarred, tumor-adjacent tissues, the cells displayed increased deformability and varied responses. Surprisingly, the typically less mobile OVCAR-3 cells became more migratory than SK-OV-3 cells in these conditions.
“The stiffness of the microenvironment has long been considered a critical factor in aggravating cancer migration. However, it was unexpected to see the epithelioid OVCAR-3 cells surpass mesenchymal SK-OV-3 cells in migratory activity on stiff matrices,” said Madumitha Suresh, a former MTech student in the Department of Bioengineering and the study’s first author.
The researchers also discovered a unique “slip” movement in OVCAR-3 cells on stiff surfaces. Unlike most cells, where movement aligns with their shape, these cells exhibited a disjointed motion, sliding or slipping in unpredictable directions.
To better understand these behaviors, the team developed a novel software toolkit combining Shannon entropy, a measure of randomness, with traditional movement- and shape-based metrics. This approach enabled the researchers to track and quantify live cell behavior over time, providing a more accessible and precise method for analyzing cell movement compared to existing mathematical or machine-learning models.
“This toolkit helped us identify how epithelioid cells adopt a less constrained relationship between their shape and motion, enabling them to migrate in diverse and unexpected ways,” explained Associate Professor Ramray Bhat from the Department of Developmental Biology and Genetics, who is the study’s corresponding author.
Looking ahead, the researchers plan to explore the collective dynamics of cancer cells in complex 3D environments, which could deepen understanding of ovarian cancer—a disease notorious for its rapid metastasis and high mortality.
These findings offer a crucial step toward unraveling the intricate mechanisms of metastasis, potentially paving the way for novel therapeutic approaches.