Vibrant green leaves sprout from tall fragrant plants sitting neatly in two rows of terracotta pots in Valerie Sponsel’s UTSA biology laboratory. One floor just above her is the chemistry lab of Francis Yoshimoto, who is extracting the plant’s leaves for medicinal compounds. Soon, the researchers will meet with UTSA researcher Annie Lin, who will test the extracted compounds on cancer cells.
The plant is Artemisia annua, or Sweet Annie, and it contains medicinal compounds. UTSA researchers are studying the plant to understand the bioactive properties of one of these compounds, Arteannuin B, in cancer cells and COVID, the disease caused by the virus, SARS-CoV-2.
“Around 50% of prescription drugs are derived from natural products. They’re made by plants, fungi or bacteria. Half of these drugs originated in plants. That’s astonishing when you think of all the medicines that exist in the world,” Sponsel said. “Different plants produce different medicinal compounds. As far as cancer is concerned, there are several types of compounds that have always existed but have only been discovered in the last half century. There’s never going to be one compound that treats all cancers, so that is why research continues.”
Sweet Annie has been used in traditional Chinese medicine for over 2,000 years. The plant produces artemisinin, which contains an endoperoxide, used for the treatment of malaria. Its leaf extracts have been used to treat a variety of other diseases, including cancer and COVID-19. Coffee infused with Sweet Annie is the focus of a current cancer-related clinical trial while the plant extract infused in tea has been used in Africa to potentially combat COVID.
Yet, until recently, researchers haven’t clearly understood how exactly the plant’s compounds work. Sponsel, Yoshimoto and Lin have been the first to demonstrate the mechanism of one of these molecules through their interdisciplinary work in biochemistry, chemistry, and biology.
“We’re in the first phases of studying the mechanism of action of Sweet Annie’s medicinal compounds to decide how to best deliver them and target therapy,” said Lin, an associate professor in the UTSA Department of Integrative Biology and the Department of Neurosciences, Developmental and Regenerative Biology. “We can be more specific. We can lower the concentration to directly target tumors. Right now, we’re looking at how to encapsulate the compound into various concentrations that will specifically target areas in need of the treatment.”
The research has been a collaborative effort with Mitchel S. Berger, professor and director of the University of California San Francisco (UCSF) Brain TumorCenter, and was recently published in Journal of Natural Products. Berger provided the resources for primary glioblastoma cells from the UCSF Brain Tumor Tissue Bank.
“We used methanol as the solvent to extract the compound, and that’s where I got the idea that this must be how it works in biological systems,” explained Yoshimoto, a UTSA assistant professor in chemistry.
Kaitlyn Varela, a doctoral student in Yoshimoto’s lab, fractionated and characterized the Sweet Annie leaf extracts by using NMR spectroscopy and liquid chromatography-mass spectrometry.
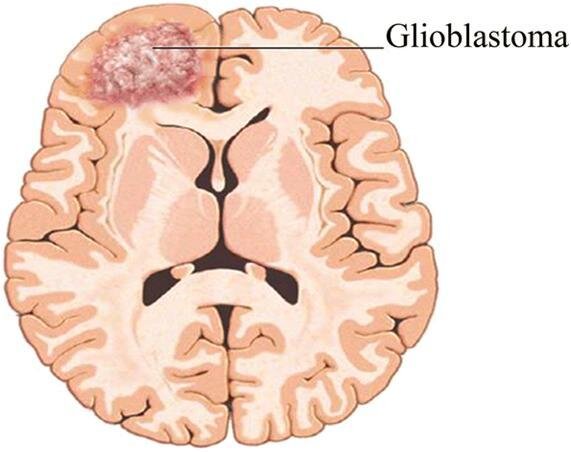
The researchers tested the fractions for cytotoxic activity (how toxic a substance is to cells) against glioblastoma (GBM) cells, a malignant form of brain tumor. Then they purified the fractions to identify and test their individual components against cancer cells one by one. Throughout the process, arteannuin B consistently demonstrated cytotoxic activity against GBM cancer cells. They believe it may inhibit the cysteine proteases (protein-degrading enzymes) that are overexpressed in cancer cells.
“We then derivatized arteannuin B by chemically reducing it, and Dr. Lin showed that the reduced form of arteannuin B was not active against GBM at the same concentration. This result informed us how arteannuin B has bioactive properties,” said Yoshimoto. “To expand on our results, Kaitlyn showed that arteannuin B hinders the activity of SARS-CoV-2 main protease and caspase-8. Both enzymes are cysteine proteases.”
Yoshimoto added, “We want to know how this works so that we can give medicine to somebody in a smart way. All of our bodies are different. Cancer, for example, overexpresses certain genes and if you know what gene is being expressed then you can target it and block the activity of its protein product with a drug. One specific example is tamoxifen, which is a prodrug that is metabolized to its active form, endoxifen, by a key enzyme in the body, cytochrome P450 2D6. Endoxifen blocks the activity of the estrogen receptor, which some estrogen-dependent breast cancers overexpress and need to grow. However, some people have fewer active forms of P450 2D6, so tamoxifen would not be effective in treating their estrogen-dependent cancers. To be able to understand the mechanism of how medicines work is really powerful because it enables medication to be given more effectively.”