
Recently, scientists in Australia have made significant progress in understanding fibrosis by identifying a positive feedback loop that sustains this damaging condition in organs with extensive fibrotic scarring. Dr. Ziyan Pan and colleagues from the Westmead Institute for Medical Research near Sydney have pinpointed a promising new target for drug therapy by isolating and defining the molecular mechanisms of this feedback loop.
In a study published, Pan and his team highlighted that identifying this feedback loop was crucial for understanding how fibrosis persists and contributes to organ damage, often with deadly consequences. They explained that fibrosis underlies many chronic diseases that are challenging to treat. While the cytokine known as transforming growth factor-β (TGFβ) plays a key role in regulating fibrosis, its involvement in other essential biological processes makes it an unlikely candidate for drug development. “TGFβ drives fibrosis and disease progression in a number of chronic disorders, but targeting this ubiquitously expressed cytokine may not yield a viable and safe antifibrotic therapy,” Pan wrote in the journal.
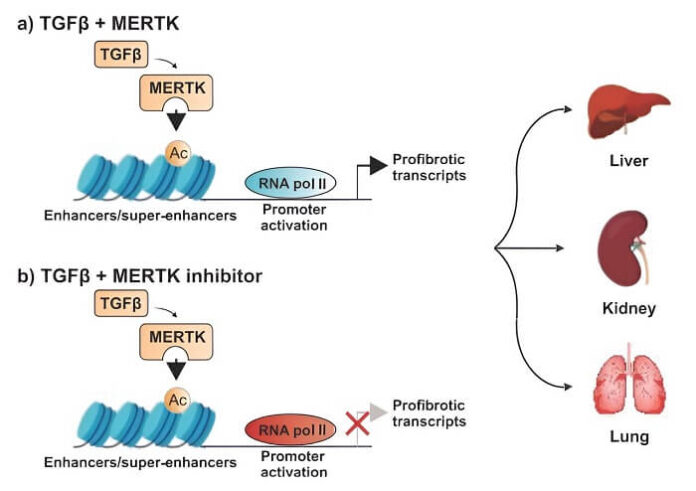
As reported by medicalxpress.com, to find alternative strategies to inhibit TGFβ signaling, Pan and his colleagues conducted experiments using human hepatic stellate cells and macrophages, as well as mouse models of liver, kidney, and lung fibrosis. They discovered that the enzyme MERTK was present at high levels in these fibrotic organs. They also observed that liver biopsy samples from patients with advanced stages of fibrotic fatty liver disease exhibited elevated levels of MERTK. This led the researchers to investigate whether blocking MERTK could interrupt the fibrosis-promoting feedback loop and offer a potential treatment strategy, irrespective of the affected organ.
In further studies using mouse models of liver, kidney, and lung fibrosis, they found that MERTK not only promoted TGFβ expression but also sustained TGFβ signaling, creating a positive feedback loop that fueled fibrosis. By inhibiting MERTK, they were able to disrupt this loop, thereby reducing fibrosis. “MERTK increased transcription of genes regulating fibrosis by modulating chromatin accessibility and RNA polymerase II activity,” Pan noted. “In each of the three mouse models, disrupting the fibrosis-promoting signaling loop by reducing MERTK expression reduced organ fibrosis.”
The researchers then tested an experimental drug, a MERTK inhibitor called UNC569, on fibrotic cells. The investigational drug significantly reduced fibrosis in the liver, kidneys, and lungs of mouse models when administered early in the disease process. Remarkably, the drug also reversed liver fibrosis in mice with established liver damage.
“Pharmacological inhibition of MERTK reduced fibrosis in these mouse models either when initiated immediately after injury or when initiated after fibrosis was established,” Pan confirmed. “Together, these data suggest that MERTK plays a role in modulating organ fibrosis and may be a potential target for treating fibrotic diseases.”
While it remains uncertain whether UNC569 will advance to clinical trials for human testing, Pan and his team have demonstrated that fibrosis is perpetuated by a common positive feedback loop, which can be interrupted with targeted pharmaceutical intervention. “These findings not only expand our understanding of MERTK’s role but also reveal new regulatory molecules that influence TGF-β activity during fibrosis,” Pan concluded.