Indian Generic Drugs Linked to More Severe Adverse Events
A new study finds Indian-made generic drugs linked to 54% more severe adverse events. These include hospitalization, disability, and death. The risk is higher than with U.S.-made generics. The findings were primarily driven by ‘mature generic drugs’—those that have been on the market for a long time.
Manufacturing Location Matters
John Gray, co-author and professor of operations at The Ohio State University’s Fisher College of Business, emphasized the impact of manufacturing location on drug safety. “Where generic drugs are manufactured can make a significant difference,” he noted.
Generic drugs contain the same chemical composition as their brand-name counterparts and offer similar dosage, safety, and effectiveness. These drugs enter the market after patents for the original brand expire.
Study Highlights Differences in Drug Quality
The results, published in the journal Production and Operations Management, indicate that not all generic drugs are equal, despite common claims that they are. Gray highlighted that regulatory differences between emerging economies like India and advanced economies like the United States contribute to variations in quality assurance practices.
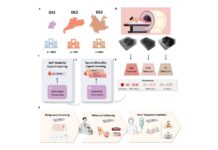
As reported by business-standard.com, the researchers analyzed 2,443 generic drugs produced in both the US and emerging economies. Since 93% of generic drugs from emerging economies are manufactured in India, the study primarily focused on India-made generics.
Adverse Events in India-Made vs. US-Made Drugs
The team compared the frequency of adverse events reported for generic drugs made in India to those reported for equivalent drugs made in the US. Their analysis showed that severe adverse events were 54.3% more common in generic drugs from emerging economies than in those from advanced economies. The researchers concluded that mature India-made generics entirely drove this effect compared to their US-made counterparts.
Cost Pressures and Drug Quality
Gray explained that as older drugs become more affordable, competition increases. This leads to cost-cutting measures that may impact operations and supply chains, ultimately compromising drug quality.
Study’s Unique Strengths
This research is significant as it is the first large-scale study to link generic drugs directly to their manufacturing plants. Another key strength of the study is the comparison of “pharmaceutically equivalent” drugs—those with the same active ingredients, dosage, and method of administration.
No Call to Stop Overseas Production
Gray cautioned against interpreting the findings as a reason to halt overseas production of generic drugs. The US Food and Drug Administration (FDA) monitors adverse events through its FAERS database. This system ensures ongoing oversight of drug safety worldwide.
By shedding light on these manufacturing differences, this study highlights the need for stringent quality control measures to ensure patient safety in the Indian generic drug market.