In a significant breakthrough for dementia care, US regulators have approved the first blood test to help diagnose Alzheimer’s disease. The move could dramatically improve how patients are identified and treated for the neurodegenerative condition that currently affects nearly 7 million Americans.
A Simpler, Less Invasive Path to Diagnosis
The newly approved test, Lumipulse, is developed by Fujirebio Diagnostics Inc., a unit of Japan’s H.U. Group Holdings Inc. The US Food and Drug Administration (FDA) cleared the test for use in individuals aged 55 and older who show signs of cognitive impairment.
Unlike current diagnostic options, which involve expensive PET scans or invasive spinal fluid tests, Lumipulse only requires a simple blood draw, making it a much more accessible tool for both patients and healthcare providers.
Targeting a Key Alzheimer’s Marker: Amyloid
The test detects levels of amyloid beta, a protein that builds up in the brain and is considered a hallmark of Alzheimer’s disease. While it is not intended for general screening, it can aid clinicians in determining whether amyloid is likely present, allowing for further evaluation and potentially faster access to treatment.
Studies show that amyloid accumulation can begin years before symptoms appear, underscoring the importance of early detection tools like this one.
Expert Reactions: “A Major Milestone”
Dr. Howard Fillit, co-founder and chief science officer at the Alzheimer’s Drug Discovery Foundation, hailed the US FDA approval as a “game changer.” “The ability to diagnose Alzheimer’s earlier with a simple blood test, like we do for cholesterol, is a game changer,” Fillit said. “It allows more patients to receive treatments that could significantly slow or even prevent disease progression.”
Easing Access to Alzheimer’s Treatments
Until now, patients seeking treatment with recently approved Alzheimer’s drugs—such as Leqembi from Eisai and Biogen, or Kisunla from Eli Lilly—have faced obstacles due to diagnostic requirements. PET scans are costly and not widely available, and spinal fluid tests can be invasive and uncomfortable.
By reducing the need for these procedures, Lumipulse could accelerate the rollout of these therapies. According to Evan Seigerman, an analyst at BMO Capital Markets, the approval offers a “much-needed win” for pharmaceutical companies struggling with adoption barriers. “Not a sea change, but today’s announcement could start to help these franchises gain some more momentum,” he wrote in a note to clients.
Important Caveats and Next Steps
The US FDA emphasized that doctors should not use Lumipulse as a standalone diagnostic tool despite its promise. Because false positives or false negatives still pose a risk, clinicians must interpret results alongside additional clinical assessments and tests. The agency also noted that specialists should use the test in specialized care settings, and it remains unclear when it will become widely available or how much it will cost.
A Step Toward Earlier, More Equitable Care
As the healthcare system continues to prioritize early detection and targeted treatment, the approval of a blood-based diagnostic for Alzheimer’s marks a pivotal shift. As reported by msn.com, with broader access and less invasive methods, more patients may be able to receive a diagnosis—and begin treatment—sooner and with fewer hurdles.
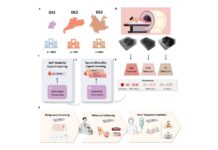
Telangana Taps AI to Revolutionise Cancer Screening in Public Healthcare
Telangana’s health department boldly proposes integrating artificial intelligence (AI) into public healthcare to modernize cancer diagnostics. This tech-driven initiative aims to enhance early detection and diagnosis, making cancer care faster, more accurate, and widely accessible.
Precision Imaging for Early Detection
The pilot project, set to launch in three districts, will harness high-resolution imaging and AI-powered analysis to detect early signs of cancer. When the AI identifies any irregularities, it will immediately relay the results to oncologists or specialist doctors for further evaluation—significantly reducing diagnosis time.
Focus on High-Burden Cancers
The program will initially target oral, breast, and cervical cancers, which contribute significantly to India’s cancer burden. Based on the pilot’s success, the government plans to scale the initiative statewide and install AI systems across all medical colleges.
Software Development and Specialised Training Underway
The MNJ Cancer Institute is currently leading the software development for the project. To ensure smooth adoption, medical staff will receive specialised training in AI-supported diagnostic procedures. This training will empower frontline workers to effectively interpret AI-generated results and deliver better patient care.
Day Care Centres and Decentralised Treatment
Alongside the AI rollout, the government is considering establishing dedicated day care cancer screening centres in every district. The government will extend chemotherapy services to Siddipet, Sircilla, and Adilabad, bringing life-saving treatment closer to remote and underserved communities.
Central Funding and Bridging the Radiologist Gap
With growing national interest in AI-led healthcare transformation, the project may receive dedicated central funding. Officials believe that deploying AI will mitigate the shortage of radiologists in the state, enabling faster, more accurate diagnostics and improving patient outcomes.
A Vision for the Future of Cancer Care
Telangana combines technological innovation with strategic public health planning to set the stage for faster, earlier, and more equitable cancer detection. If successful, the project could become a model for AI-driven healthcare across India.