
A team from the International Research Center for Medical Sciences (IRCMS) at Kumamoto University has discovered a novel molecular mechanism connecting fetal anemia to disrupted intracellular iron distribution. This disruption stems from impaired mitochondrial protein synthesis, offering new insights into iron-related diseases and potential therapeutic avenues.
Study Led by Experts in Mitochondrial Research
Dr. Tatsuya Morishima, a lecturer and Wakakusu researcher at IRCMS, and Professor Hitoshi Takizawa led the groundbreaking research. Their findings were recently published in the journal Science Advances.
Mitochondrial Protein Synthesis: A Vital but Overlooked Process
Although most proteins are synthesized in the cytosol, a small yet essential fraction is produced within mitochondria—critical for cellular energy generation. Mitochondrial protein synthesis depends heavily on the chemical modification of mitochondrial tRNAs, a process mediated by specialized enzymes.
One such enzyme is MTO1. It plays a vital role by modifying mitochondrial tRNAs to enable efficient protein production. While mutations in the MTO1 gene are known to cause severe anemia in humans, scientists had not fully understood whether defective mitochondrial protein synthesis directly leads to blood disorders.
Creating a Mouse Model to Investigate the Link
To explore this question, the IRCMS team developed a mouse model with the MTO1 gene specifically knocked out in hematopoietic (blood-forming) cells. All mice with the knockout died before birth, displaying signs of severe fetal anemia. Since blood formation occurs primarily in the fetal liver, researchers focused their analysis on fetal liver cells.
Disrupted Mitochondrial Function and Iron Imbalance
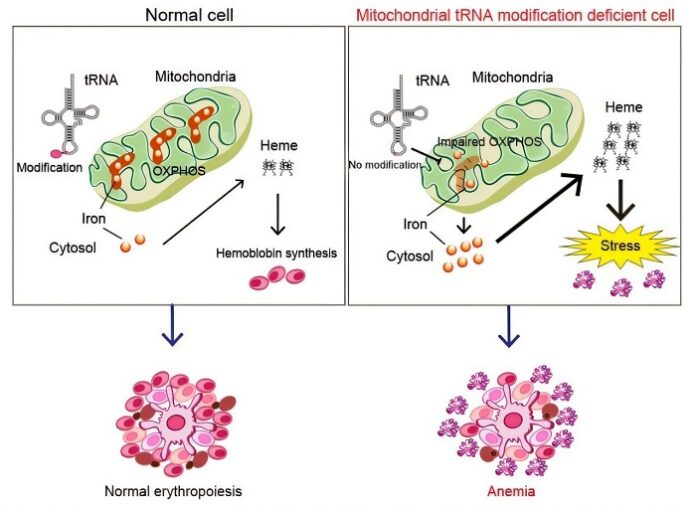
As reported by medicalxpress, their investigation revealed that the knockout cells showed a significant defect in the formation of mitochondrial oxidative phosphorylation (OXPHOS) complexes. These complexes, essential for energy production, incorporate various forms of iron into their structure. However, in the absence of MTO1, mitochondrial iron levels plummeted, while cytosolic iron levels surged.
This iron imbalance triggered excessive heme production—a key component of hemoglobin. However, instead of correcting the anemia, the surplus heme caused cellular stress in red blood cells, exacerbating the condition.
A New Mechanism Behind Fetal Anemia
The study sheds light on a previously unrecognized role of mitochondrial protein synthesis in regulating intracellular iron distribution. Proper formation of OXPHOS complexes appears crucial not only for energy production but also for maintaining iron balance within cells. When this system fails, it can lead to lethal fetal anemia.
Implications for Future Research and Treatment
These findings mark a significant step forward in our understanding of how mitochondrial dysfunction can cause hematological disorders. They open new possibilities for therapeutic strategies aimed at treating or preventing anemia by targeting mitochondrial protein synthesis and iron regulation pathways.